��Ŀ����
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�á�

��1��װ��B����֮һ��Ϊ�˳�ȥ�����е�����HCl��ʢװ��Һ���Լ�Ϊ__________��
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η����������ȷ����__________ (����ĸ���) ��
��� | �� | �� | �� |
a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��3��D�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________����װ��D�е���Һ����װ��E�У���Һ��Ϊ���㣬�ϲ���Ϻ�ɫ��Ҫ������Ϻ�ɫ��Һ����ʹ�õIJ��������Dz��������ձ���_____________��
��4��װ��F�з�Ӧ�Ļ�ѧ����ʽΪ_____________��
��5�������ʵ��ʹ�õ�Ũ������������Ϊ36.5%���ܶ�Ϊ1.15 g/cm3������������ʵ���Ũ��Ϊ_____mol��L-1������250 mLˮ���ƣ���Ӧ�ܽ�����HCl����ԼΪ_____________L���������3λ��Ч���֣���
���𰸡�����ʳ��ˮ d Cl2+2NaBr=2NaCl+Br2 ��Һ©�� Cl2+2NaOH=NaCl+NaClO+H2O 11.5 88.2
��������
(1)����ʳ��ˮ�����������е�HCl��(2)Ϊ����֤�����Ƿ����Ư���ԣ�I�м���ʪ�����ɫ������IIΪU�ܣ��ɼ�������������õ������Cl2��III�м���������ɫ����������֤��Cl2�Ƿ����Ư���ԣ�(3) D�л���ͨ����������ʱ���������廯�Ʒ�Ӧ�����Ȼ��ƺ��嵥�ʣ�������ӦCl2+2NaBr=Br2+2NaCl���÷�Һ�����뻥�����ݵ�Һ������4��װ��F�����������������������Ȼ�����������������5������![]() ������������ʵ���Ũ�ȣ�����
������������ʵ���Ũ�ȣ�����![]() ��������HCl����������
��������HCl����������
(1)����ʳ��ˮ�����������е�HCl��װ��B�е��Լ��DZ���ʳ��ˮ����2��Ϊ����֤�����Ƿ����Ư���ԣ�I�м���ʪ�����ɫ������IIΪU�ܣ��ɼ�������������õ������Cl2��III�м���������ɫ����������֤��Cl2�Ƿ����Ư���ԣ���ѡd��(3) D�л���ͨ����������ʱ���������廯�Ʒ�Ӧ�����Ȼ��ƺ��嵥�ʣ�������ӦCl2+2NaBr=Br2+2NaCl���÷�Һ�����뻥�����ݵ�Һ����Ҫ������Ϻ�ɫ��Һ����ʹ�õIJ��������Dz��������ձ��ͷ�Һ©������4��װ��F�����������������������Ȼ�����������������Ӧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O����5��![]() =11.5 mol��L-1������Ҫ�����HCl��������ΪVL����HCl�����ʵ�����
=11.5 mol��L-1������Ҫ�����HCl��������ΪVL����HCl�����ʵ�����![]() ����Һ��������
����Һ��������![]() ����Һ�����
����Һ�����![]() ������
������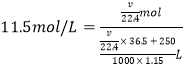 ����V= 88.2L��
����V= 88.2L��

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�