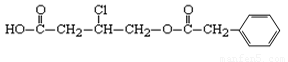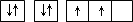��Ŀ����
����ѡ�����и����з��������ѡ�
��1�����в����ܴ��ڵ��л�����
A��2�������� B��2��3�����ȡ�2��2����������
C��3���塪3���һ����� D��2��2��3��3���ļ�����
��2������KOH�Ĵ���Һ�з�����ȥ��Ӧ����
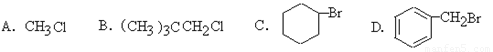
��3��ij�л�����ܷ���������Ӧ�����ܷ�����ԭ��Ӧ������������ͻ�ԭ�����ܷ���������Ӧ�������ɵ���Ҳ�ܷ���������Ӧ������л���Ľṹ��ʽ��
A��CH3OH B��HCHO
C��HCOOH D��HCOOCH3
��12�֣��л���A�Ľṹ��ʽΪ��
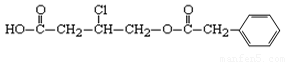
�ݴ˻ش��������⣺
��1��A�ķ���ʽΪ__________��
��2��A��NaOHˮ��Һ�м��ȣ����ữ�õ��л���B��D��D�Ƿ����廯�������1 mol A������Ӧʱ��������� mol NaOH��
��3��B��һ�������·���������Ӧ������ij��Ԫ����������Ԫ�����Ľṹ��ʽΪ ��
��4��д��������ֻ��һ��ȡ���������������D������ͬ���칹��Ľṹ��ʽ�� ��
��(1)B (2)C (3)B
��1��C12H13O4Cl ��2�֣���2��3��2�֣���3�� 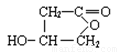 ��2�֣�
��2�֣�
��4��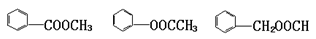 ����2�֣�
����2�֣�
��������
�����������1������̼ԭ�ӵļ۵�����4��������̼ԭ�Ӳ������γ�5�����ۼ���B����ȷ���������ȷ�ģ���ѡB��
��2��±����������ȥ��Ӧ����������±��ԭ�����ӵ�̼ԭ�ӵ���λ̼ԭ�ӱ��뺬����ԭ�ӣ��ݴ˿��ж�ѡ��C�ɷ�����ȥ��Ӧ����ѡC��
��3���ܷ���������Ӧ����һ���Ǽ����γɵ��������Ը��л����Ǽ�ȩ����ѡB��
��1������A�ṹ��ʽ������̼ԭ�ӵļ۵�������֪������ʽΪC12H13O4Cl��
��2��A�к���1���Ȼ���1����ԭ�Ӻ�1�������������Ҫ3mol�������ơ�
��3��A���������Ƶ���Һ�м��ȣ�����ˮ�ⷴӦ��������ֱ���HOOC
CH2CHOHCH2OH��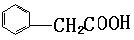 ������D�Ƿ����廯�������B��HOOC CH2CHOHCH2OH��B�к����Ȼ����ǻ����ɷ��������ڵ�������Ӧ�������γɵ���Ԫ������
������D�Ƿ����廯�������B��HOOC CH2CHOHCH2OH��B�к����Ȼ����ǻ����ɷ��������ڵ�������Ӧ�������γɵ���Ԫ������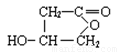 ��
��
��4���������࣬ʯī���뺬�����������Ը���D�Ľṹ��ʽ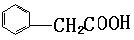 ��֪����ͬ���칹����
��֪����ͬ���칹����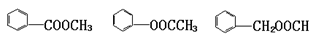 ��
��
���㣺�л���ѧ����֪ʶ���漰�л���ṹ�������������ŵ����ʵ�

 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�[��ѧ����ѡ�����ʽṹ������]��30�֣�
��18�֣���ѡ�����и����з��������ѡ�
��1�����������У����ں��й��ۼ������Ӿ�����
A��CsCl B��KOH C��H2O D��H2
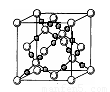
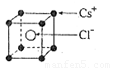
��2����֪CsCl������ܶ�Ϊ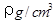 ��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ
��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ 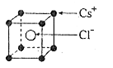
A�� g/mol B��
g/mol B�� g/mol
g/mol
C��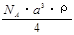 g/mol D��
g/mol D��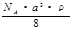 g/mol
g/mol
��3����֪���������ͨʽXOm(OH)n����ʾ����X��S��m=2��n=2�������ʽ�Ӿͱ�ʾH2SO4��һ����ԣ���ʽ��m��ֵԽ�ú����������Խǿ�����и���������������ǿ����
A��HMnO4 B��H2SeO3 C��H3BO3 D��H3PO4
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ԭ�ӵĵ����Ų�ͼΪ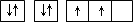 |
| B | �����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
| C | ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
| D | ������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
| E | ԭ��������D������ |
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��
��3��E�����ڱ��е�λ���� ��ECl3����B��C���⻯���γ���λ��Ϊ6��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
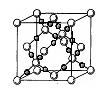
��4��AC2�ڸ��¸�ѹ�����γɵľ�������ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��
��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��
[��ѧ����ѡ�����ʽṹ������]��30�֣�
��18�֣���ѡ�����и����з��������ѡ�
��1�� ���������У����ں��й��ۼ������Ӿ�����
A��CsCl B��KOH C��H2O D��H2
��2����֪CsCl������ܶ�Ϊ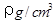 ��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ
��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ
A�� 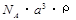 g/mol B��
g/mol B��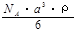 g/mol
g/mol
C��  g/mol D��
g/mol D��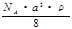 g/mol
g/mol
��3����֪���������ͨʽXOm(OH)n����ʾ����X��S��m=2��n=2�������ʽ�Ӿͱ�ʾH2SO4��һ����ԣ���ʽ��m��ֵԽ�ú����������Խǿ�����и���������������ǿ����
A��HMnO4 B��H2SeO3 C��H3BO3 D��H3PO4
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
A |
ԭ�ӵĵ����Ų�ͼΪ |
|
B |
�����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
|
C |
ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
|
D |
������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
|
E |
ԭ��������D������ |
��������������ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��
��3��E�����ڱ��е�λ���� ��ECl3����B��C���⻯���γ���λ��Ϊ6��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
��4��AC2�ڸ��¸�ѹ�����γɵľ�������ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��
��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��