��Ŀ����
��һ��д���йط�Ӧ�����ӷ���ʽ��
��1������������Һ��̼��������Һ�ķ�Ӧ______��
��2��������Ͷ��ϡ�����з�Ӧ______��
��3����Na2CO3��Һ�м�������ϡ����______��
��4����̼�������Һ�м��������ռ���Һ______��
������ij��ɫ��Һ����Na+��Ag+��Ba2+��Al3+��Al
��Mn
��C
��S
�е���������ɣ�ȡ����Һ��������ʵ�飺
��ȡ������Һ������������ᣬ���������ɣ����õ���Һ��
���ڢ�������Һ���ټ������̼�������Һ�����������ɣ�ͬʱ������ɫ�����ף�
���ڢ�������Һ�м������Ba��OH��2��Һ��Ҳ���������ɣ����а�ɫ������������
��������ʵ��ش��������⣺
��1����Һ��һ�������ڵ�������______��
��2��һ�����ڵ�������______��
��1������������Һ��̼��������Һ�ķ�Ӧ______��
��2��������Ͷ��ϡ�����з�Ӧ______��
��3����Na2CO3��Һ�м�������ϡ����______��
��4����̼�������Һ�м��������ռ���Һ______��
������ij��ɫ��Һ����Na+��Ag+��Ba2+��Al3+��Al
| O | -2 |
| O | -4 |
| O | 2-3 |
| O | 2-4 |
��ȡ������Һ������������ᣬ���������ɣ����õ���Һ��
���ڢ�������Һ���ټ������̼�������Һ�����������ɣ�ͬʱ������ɫ�����ף�
���ڢ�������Һ�м������Ba��OH��2��Һ��Ҳ���������ɣ����а�ɫ������������
��������ʵ��ش��������⣺
��1����Һ��һ�������ڵ�������______��
��2��һ�����ڵ�������______��
��һ����1������������Һ��̼��������Һ�ķ�Ӧ��ʵ��ΪH+��HCO3-��Ӧ�������ӷ�ӦΪH++HCO3-�TCO2��+H2O���ʴ�Ϊ��H++HCO3-�TCO2��+H2O��
��2��������Ͷ��ϡ�����з�Ӧ��ʵ��ΪFeS��H+��Ӧ�������ӷ�ӦΪFeS+2H+�TFe2++H2S�����ʴ�Ϊ��FeS+2H+�TFe2++H2S����
��3����Na2CO3��Һ�м�������ϡ��������NaHCO3��NaCl����Ӧʵ��ΪCO32-��H+��Ӧ����HCO3-�������ӷ�ӦΪCO32-+H+�THCO3-���ʴ�Ϊ��CO32-+H+�THCO3-��
��4����̼�������Һ�м��������ռ���Һ�ķ�Ӧʵ��ΪCa2+��HCO3-��OH-��Ӧ�������ӷ�ӦΪCa2++HCO3-+OH-�TCaCO3��+H2O���ʴ�Ϊ��Ca2++HCO3-+OH-�TCaCO3��+H2O��
��������MnO4-����Һ��Ϊ��ɫ������ɫ��Һ��һ��û��MnO4-���ɢٿ�֪һ������CO32-����û��Ag+��Ba2+��Al3+�����ڢ�������Һ���ټ������̼�������Һ��֪���ɵ�����Ϊ������̼��������ΪAl��OH��3������Һ��һ������AlO2-�����ڢ�������Һ�м������Ba��OH��2��Һ��Ҳ���������ɣ����а�ɫ������������֪�����ɵ�����Ϊ��������������һ������BaCO3�����ܺ���BaSO4������Һ�п��ܺ���SO42-������Һ���Ե��ԣ���һ�����ڵ�������ΪNa+��
��1��������������Һ��һ�������ڵ�����ΪAg+��Ba2+��Al3+��MnO4-���ʴ�Ϊ��Ag+��Ba2+��Al3+��MnO4-��
��2��һ�����ڵ�����ΪNa+��CO32-��AlO2-���ʴ�Ϊ��Na+��CO32-��AlO2-��
��2��������Ͷ��ϡ�����з�Ӧ��ʵ��ΪFeS��H+��Ӧ�������ӷ�ӦΪFeS+2H+�TFe2++H2S�����ʴ�Ϊ��FeS+2H+�TFe2++H2S����
��3����Na2CO3��Һ�м�������ϡ��������NaHCO3��NaCl����Ӧʵ��ΪCO32-��H+��Ӧ����HCO3-�������ӷ�ӦΪCO32-+H+�THCO3-���ʴ�Ϊ��CO32-+H+�THCO3-��
��4����̼�������Һ�м��������ռ���Һ�ķ�Ӧʵ��ΪCa2+��HCO3-��OH-��Ӧ�������ӷ�ӦΪCa2++HCO3-+OH-�TCaCO3��+H2O���ʴ�Ϊ��Ca2++HCO3-+OH-�TCaCO3��+H2O��
��������MnO4-����Һ��Ϊ��ɫ������ɫ��Һ��һ��û��MnO4-���ɢٿ�֪һ������CO32-����û��Ag+��Ba2+��Al3+�����ڢ�������Һ���ټ������̼�������Һ��֪���ɵ�����Ϊ������̼��������ΪAl��OH��3������Һ��һ������AlO2-�����ڢ�������Һ�м������Ba��OH��2��Һ��Ҳ���������ɣ����а�ɫ������������֪�����ɵ�����Ϊ��������������һ������BaCO3�����ܺ���BaSO4������Һ�п��ܺ���SO42-������Һ���Ե��ԣ���һ�����ڵ�������ΪNa+��
��1��������������Һ��һ�������ڵ�����ΪAg+��Ba2+��Al3+��MnO4-���ʴ�Ϊ��Ag+��Ba2+��Al3+��MnO4-��
��2��һ�����ڵ�����ΪNa+��CO32-��AlO2-���ʴ�Ϊ��Na+��CO32-��AlO2-��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
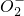 ��Mn
��Mn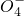 ��C
��C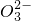 ��S
��S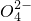 �е���������ɣ�ȡ����Һ��������ʵ�飺
�е���������ɣ�ȡ����Һ��������ʵ�飺