��Ŀ����
��֪NH3��HCl��������������Ȫʵ������塣����ͬ��ͬѹ���õ������һ����ƿ�ռ���NH3����һ���ռ�HCl����δ������Ȫʵ��ֹͣ��������ƿ����Һ�Ĺ�ϵ��(���������ʵ���ɢ����ֹʱҺ��߶ȵ�Ӱ��)( )
| A�����ʵ����ʵ���Ũ����ͬ�����ʵ�����������ͬ |
| B�����ʵ�����������ͬ�����ʵ����ʵ���Ũ�Ȳ�ͬ |
| C�����ʵ����ʵ���Ũ�Ⱥ����ʵ�������������ͬ |
| D�����ʵ����ʵ���Ũ�Ⱥ����ʵ�������������ͬ |
A
����

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ� ��
| A��1L1mol?L-1��NH4Cl��Һ��һ������NA��NH4+ |
| B�����³�ѹ�£�22.4L��ϩ�к����ۼ���Ϊ5 NA |
| C��6.8g���ڵ�KHSO4�к���0.05 NA�������� |
| D��1mol�������lmo1�Ҵ���Ũ��������·�Ӧ������NA��H2O |
�����ᡢ��������������ɵĻ����Һ�У�c(Al3+)="0.3" mol��L-1��c(SO42��)=0.7mol��L-1�� ��ˮ�����c(H+)=10-13 mol��L-1����c(K+)Ϊ
| A��0.15mol��L-1������ | B��0.2mol��L-1 | C��0.3mol��L-1 | D��0.4mol��L-1 |
�����йػ�ѧ�����ʾ��ȷ���ǣ� ��
A��H3O���ĵ���ʽ��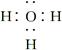 |
B����NH2�ĵ���ʽ��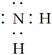 |
C��NaHS�Լ��Ե�ԭ��HS����H2O S2����H3O�� S2����H3O�� |
| D������Ķ��ȴ���������(�����������칹) |
����Ŀ���ܴﵽ����( )
| A����58��5 g NaCl����1 Lˮ�пɵ�1 mol��L��1��NaCl��Һ |
| B������״����22��4 L HCl����1 Lˮ�пɵ�1 mol��L��1���� |
| C����25��0 g��������ˮ�����100 mL��Һ������ҺŨ��Ϊ1 mol��L��1 |
| D����78 g Na2O2����ˮ�����1 L��Һ�ɵõ�Ũ��Ϊ1 mol��L��1��Һ |
V LFe2(SO4)3��Һ�к���a g SO42-��ȡ����Һ0��5V L����ˮϡ����2V L����ϡ�ͺ���Һ��Fe3�������ʵ�����Ũ��Ϊ( )
A��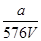 mol��L��1 mol��L��1 | B��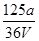 mol��L��1 mol��L��1 |
C��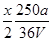 mol��L��1 mol��L��1 | D��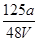 mol��L��1 mol��L��1 |
��NA��ʾ�����ӵ�������ֵ������˵����ȷ����( )
| A����״���£�0.1 mol Cl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA |
| B�����³�ѹ�£�18 g H2O�к��е�ԭ������Ϊ3NA |
| C����״���£�11.2 L CH3CH2OH�к��еķ�����ĿΪ0.5NA |
| D�����³�ѹ�£�2.24 L CO��CO2��������к��е�̼ԭ����ĿΪ0.1NA |
����˵����ȷ����(����)��
| A����������ͬ��CO��N2�����һ������ |
| B�������ͬ��CO��N2������һ����� |
| C��������ͬ���ܶȲ�ͬ��CO��N2������ԭ����Ŀһ����� |
| D��������ͬ���ܶ���ͬ��CO��N2����������������ͬ |
ij��Һ�д�����������Ũ�ȵ��������ӣ�0.2 mol��L��1 Cl����0.4 mol��L��1 SO42����0.1 mol��L��1 Al3����0.3 mol��L��1 H����M����M�������ʵ���Ũ�ȿ���Ϊ(����)��
| A��Na����0.3 mol��L��1 | B��Zn2����0.2 mol��L��1 |
| C��CO32����0.2 mol��L��1 | D��Ca2����0.1 mol��L��1 |