ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΗΏΖ÷Ή”≤ΡΝœPETΨέθΞ ς÷§ΚΆPMMAΒΡΚœ≥…¬ΖœΏ»γœ¬ΆΦΥυ ΨΘΚ
“―÷ΣΘΚΔώ. RCOORΓ·+RΓ·Γ·OH ![]() RCOORΓ·Γ·+RΓ·OH
RCOORΓ·Γ·+RΓ·OH
Δρ. ![]()
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1) AΒΡΖ÷Ή” Ϋ «___________ΓΘ
(2) EΒΡΫαΙΙΦρ Ϋ «________________Θ§Ζ¥”ΠΔόΒΡΖ¥”Πάύ–Ά «____________ ΓΘ
(3)Β»Έο÷ ΒΡΝΩΒΡGΖ÷±π”κΉψΝΩNaΓΔNaHCO3»ή“ΚΖ¥”ΠΘ§…ζ≥…ΒΡΤχΧε‘ΎœύΆ§Ή¥Ωωœ¬ΧεΜΐ±»ΈΣ______ΓΘ
(4) J÷–ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ__________ΓΘ
(5)–¥≥ωΖ¥”ΠΔίΒΡΜ·―ßΖΫ≥Χ ΫΘΚ__________________ΓΘ
(6)–¥≥ω“Μ÷÷ΖϊΚœœ¬Ν–ΧθΦΰΒΡPMAAΒΞΧεΒΡΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΘΚ_________________ΓΘ
a.ΨΏ”–PMAAΒΞΧεΒΡΥυ”–ΙΌΡήΆ≈b.Ρή”κ–¬÷Τ“χΑ±»ή“ΚΖ¥”Π≤ζ…ζ“χΨΒc.Κ§”–3÷÷≤ΜΆ§Μ·―ßΜΖΨ≥ΒΡ«β‘≠Ή”
ΓΨ¥πΑΗΓΩC2H4Br2 CH3CH(OH)CH3 œϊ»ΞΖ¥”Π 1:1 ΧΦΧΦΥΪΦϋΘ§τ»Μυ 2CH3CH(OH)CH3 +O2![]() 2CH3COCH3+2H2O HCOOCH=C(CH3)2
2CH3COCH3+2H2O HCOOCH=C(CH3)2
ΓΨΫβΈωΓΩ
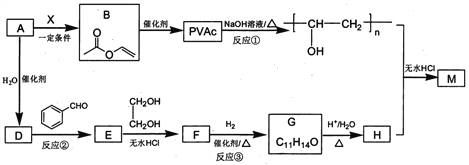
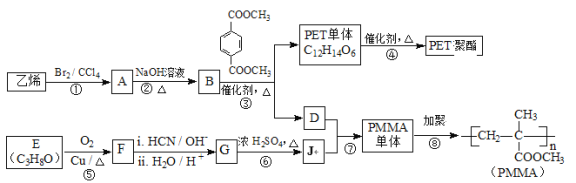
”…PMMAΒΡΫαΙΙΘ§Ω…÷ΣPMMAΒΞΧεΈΣCH2=C(CH3)COOCH3Θ§‘ρDΓΔJΖ÷±πΈΣCH2=C(CH3)COOHΓΔCH3OH÷–ΒΡ“Μ÷÷Θ§““œ©ΚΆδεΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…AΈΣCH2BrCH2BrΘ§A‘ΎNaOHΥ°»ή“ΚΓΔΦ”»»ΧθΦΰœ¬ΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…BΈΣHOCH2CH2OHΘ§ΗυΨί–≈œΔIΦΑPETΒΞΧεΖ÷Ή” ΫΘ§Ω…÷ΣPETΒΞΧεΈΣ Θ§‘ρDΈΣCH3OHΓΔJΈΣCH2=C(CH3)COOHΘ§PETΒΞΧεΖΔ…ζ–≈œΔI÷–ΫΜΜΜΖ¥”ΠΫχ––ΒΡΥθΨέΖ¥”Π…ζ≥…PET ς÷§ΈΣ
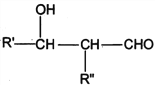
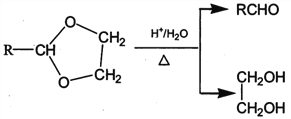
Θ§‘ρDΈΣCH3OHΓΔJΈΣCH2=C(CH3)COOHΘ§PETΒΞΧεΖΔ…ζ–≈œΔI÷–ΫΜΜΜΖ¥”ΠΫχ––ΒΡΥθΨέΖ¥”Π…ζ≥…PET ς÷§ΈΣ Θ§FΖΔ…ζ–≈œΔΔρ÷–ΒΡΖ¥”ΠΒΟΒΫGΘ§G‘Ύ≈®ΝρΥαΉς”Οœ¬ΖΔ…ζœϊ»ΞΖ¥”Π…ζ≥…JΘ§‘ρGΈΣ(CH3)2COHCOOHΘ§Ι FΈΣCH3COCH3Θ§EΈΣCH3CH(OH)CH3Θ§Ψί¥ΥΫβ¥πΓΘ
Θ§FΖΔ…ζ–≈œΔΔρ÷–ΒΡΖ¥”ΠΒΟΒΫGΘ§G‘Ύ≈®ΝρΥαΉς”Οœ¬ΖΔ…ζœϊ»ΞΖ¥”Π…ζ≥…JΘ§‘ρGΈΣ(CH3)2COHCOOHΘ§Ι FΈΣCH3COCH3Θ§EΈΣCH3CH(OH)CH3Θ§Ψί¥ΥΫβ¥πΓΘ
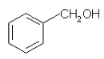
(1)ΓΔΗυΨίAΒΡΫαΙΙΦρ ΫΈΣCH2BrCH2BrΩ…÷ΣAΒΡΖ÷Ή” Ϋ «C2H4Br2Θ§Ι ¥πΑΗΈΣΘΚC2H4Br2ΘΜ
(2)ΓΔ”…Ζ÷ΈωΩ…÷ΣEΒΡΫαΙΙΦρ Ϋ «CH3CH(OH)CH3Θ§Ζ¥”ΠΔόΒΡΖ¥”Πάύ–Ά «œϊ»ΞΖ¥”ΠΘ§Ι ¥πΑΗΈΣΘΚCH3CH(OH)CH3ΘΜœϊ»ΞΘΜ
(3)ΓΔGΒΡΫαΙΙΦρ ΫΈΣ(CH3)2COHCOOHΘ§Κ§”–τ«ΜυΚΆτ»ΜυΘ§τ»ΜυΨυΡή”κNaΓΔNaHCO3»ή“ΚΘ§Εχτ«Μυ÷ΜΡή”κNaΖ¥”ΠΘ§Υυ“‘Β»Έο÷ ΒΡΝΩΒΡGΖ÷±π”κΉψΝΩNaΓΔNaHCO3»ή“ΚΖ¥”ΠΘ§…ζ≥…ΒΡΤχΧε‘ΎœύΆ§Ή¥Ωωœ¬ΧεΜΐ±»ΈΣΘΚ1:1Θ§Ι ¥πΑΗΈΣΘΚ1:1ΘΜ
(4)ΓΔ”…Ζ÷ΈωΩ…÷ΣJΒΡΫαΙΙΈΣCH2=C(CH3)COOHΘ§Υυ“‘J÷–ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣΘΚΧΦΧΦΥΪΦϋΓΔτ»ΜυΘ§Ι ¥πΑΗΈΣΘΚΧΦΧΦΥΪΦϋΓΔτ»ΜυΘΜ
(5)ΓΔΖ¥”ΠΔί”…E[CH3CH(OH)CH3]‘Ύ―θΤχΦ”»»ΒΡΧθΦΰœ¬ΖΔ…ζ¥ΦΒΡ¥ΏΜ·―θΜ·…ζ≥…F(CH3COCH3)Θ§Ι Ζ¥”ΠΔίΒΡΜ·―ßΖΫ≥Χ ΫΘΚ2CH3CH(OH)CH3 +O2![]() 2CH3COCH3+2H2OΘΜ
2CH3COCH3+2H2OΘΜ
(6)ΓΔΖϊΚœœ¬Ν–ΧθΦΰa.ΨΏ”–PMAAΒΞΧεΒΡΥυ”–ΙΌΡήΆ≈Θ§Φ¥Κ§”–ΧΦΧΦΥΪΦϋΚΆθΞΜυΘΜb.Ρή”κ–¬÷Τ“χΑ±»ή“ΚΖ¥”Π≤ζ…ζ“χΨΒΘ§ΥΒΟςΚ§”–ΦΉΥα–Έ≥…ΒΡθΞΜυΘΜc.Κ§”–3÷÷≤ΜΆ§Μ·―ßΜΖΨ≥ΒΡ«β‘≠Ή”Θ§’β―υΒΡΒΡPMAAΒΞΧεΒΡΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΈΣΘΚHCOOCH=C(CH3)2Θ§Ι ¥πΑΗΈΣHCOOCH=C(CH3)2ΓΘ

 Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΝρΥα―«Χζοß[(NH4)2Fe(SO4)2] «Ζ÷ΈωΜ·―ß÷–ΒΡ÷Ί“Σ ‘ΦΝΘ§‘Ύ≤ΜΆ§Έ¬Ε»œ¬Φ”»»Ζ÷Ϋβ≤ζΈο≤ΜΆ§ΓΘ…ηΦΤ»γΆΦ Β―ιΉΑ÷ΟΘ®Φ–≥÷ΉΑ÷Ο¬‘»ΞΘ©Θ§‘Ύ500Γφ ±ΗτΨχΩ’ΤχΦ”»»A÷–ΒΡΝρΥα―«Χζοß÷ΝΖ÷ΫβΆξ»ΪΓΘ»ΖΕ®Ζ÷Ϋβ≤ζΈοΒΡ≥…Ζ÷ΓΘ

(1)BΉΑ÷ΟΒΡΉς”Ο «________________________________ΓΘ
(2) Β―ι÷–Θ§Ιέ≤λΒΫC÷–ΈόΟςœ‘œ÷œσΘ§D÷–”–ΑΉ…Ϊ≥ΝΒμ…ζ≥…Θ§Ω…»ΖΕ®≤ζΈο÷–Ε®”–______ΤχΧε≤ζ…ζΘ§–¥≥ωD÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ____________________ΓΘ»τ»ΞΒτCΘ§ «ΖώΡήΒΟ≥ωΆ§―υΫα¬έ≤ΔΫβ ΆΤδ‘≠“ρ__________________________ΓΘ
(3)A÷–ΙΧΧεΖ÷ΫβΚσ≤ζ…ζNH3Θ§–¥≥ωΤδΝΫ÷÷”ΟΆΨ________________________________________ΓΘ
(4)A÷–ΙΧΧεΆξ»ΪΖ÷ΫβΚσ±δΈΣΚλΉΊ…ΪΖέΡ©Θ§Ρ≥Ά§―ß…ηΦΤ Β―ι―ι÷ΛΙΧΧε≤–ΝτΈοΫωΈΣFe2O3Εχ≤ΜΚ§FeOΓΘ«κΆξ≥…±μΡΎ»ίΓΘ( ‘ΦΝΘ§“«ΤςΉ‘―Γ)
Β―ι≤Ϋ÷η | ‘ΛΤΎœ÷œσ | Ϋα¬έ |
ΔΌ»Γ…ΌΝΩA÷–≤–ΝτΈο”Ύ ‘Ιή÷–Θ§Φ”»κ ΝΩœΓΝρΥαΘ§≥δΖ÷’ώΒ¥ ΙΤδΆξ»Ϊ»ήΫβ; ΔΎ______________________________________________________ | ΙΧΧε≤–ΝτΈοΫωΈΣFe2O3 |
(5) ”Ο Β―ιΒΡΖΫΖ®―ι÷ΛC»ή“ΚΚ§”–NH4+___________________________________________ΓΘ