��Ŀ����
��17�֣�����12 mol?L��1��Ũ��������0.10 mol?L��1��ϡ����500 mL���ش��������⣺
(1)��ȡŨ��������Ϊ mL�����ʵ������5mL��10mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��á�����ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ��������ҺŨ�Ƚ� (ƫ�ߡ�ƫ�͡���Ӱ��)��
(2)����ʱӦѡ�õ�����ƿ���Ϊ mL ��������ƿ����ѡ�õ�������Ҫ�� �� �� �� ��
(3)����ʱ������ȡ��Ũ�������ձ��ڱ�����ע��Լ100mLˮ������Ͻ��裬Ŀ���� ��
(4)����ȴ���������Һ�� ע�� �У�����Լ50mL����ˮϴ���ձ�2��3�Σ�ת��ҡ�ȡ�����������
(5)��ˮ����̶���  �������� �����������μ�ˮ��ʹ��Һ�İ�Һ�����ø��̶������У�������ƿ����ҡ�ȡ�
�������� �����������μ�ˮ��ʹ��Һ�İ�Һ�����ø��̶������У�������ƿ����ҡ�ȡ�
(6)��ʵ�����������,��Һ��Ũ����ƫ�� ,ƫ�ͻ��Dz���?
,ƫ�ͻ��Dz���?
| A����ˮʱԽ���̶���______ | B�����ǽ�ϴ��Һ��������ƿ______ |
C������ƿ�ڱڸ���ˮ���δ���ﴦ�� | D���ܽ��û����ȴ����ж��� |

����

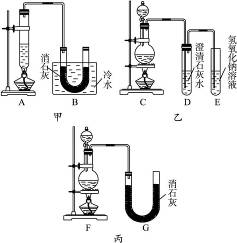
��������ͬѧ�ֱ��������ʵ��װ�����£�
(1)��ӣ�a.�������Ʒ�Ӧ���ʣ�b.�����Ʒ�Ӧ���ʣ�c.�и���Ӧ������d.�ɷ�ֹ����Ӧ������e.��Ⱦ������f.�ɷ�ֹ��Ⱦ������������������ס��ҡ�������װ�õ���ȱ���������ۣ���ѡ�������ĿҪ���ѡ�����ڿո��ڡ�
װ�� | �ŵ� | ȱ�� |
�� |
|
|
�� |
|
|
�� |
|
|
(2)ͼ�м���A��B��������ɣ�����C��D��E��������ɣ�����F��G��ɣ��������ס��ҡ�������װ����ѡ�������IJ���(�����������ҵķ���)��װһ�����Ƶ�ʵ��װ��(����ѡ���ֵı��)��_____________________��
(3)ʵ��������12 mol��L-1��Ũ����100 mL��������MnO2��Ӧ����������Ca(ClO)2�����ʵ�����С��0.15 mol������ܵ���Ҫԭ����____________________________(�ٶ�������Ӧ����Ӧ�����������Ӧ����)��
ͼ6-31
ʵ������Ũ���ᡢMnO2������Cl2������Cl2��Ca(OH)2��Ӧ������Ư�ۣ�����֪��Ӧ��2Cl2+2Ca(OH)2====Ca(ClO)2+CaCl2+2H2O,�¶��Ը���������Ӧ��6Cl2+6Ca(OH)2![]()
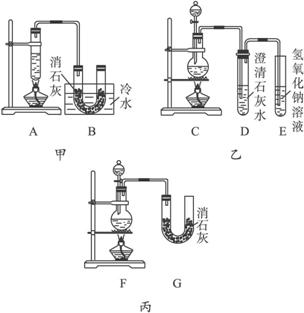
Ca(ClO3)2+5CaCl2+6H2O����������ͬѧ�ֱ���Ƶ�����ʵ��װ����ͼ��ʾ��

(1)a.�������Ʒ�Ӧ���ʣ�b.�����Ʒ�Ӧ���ʣ�c.�и���Ӧ������d.�ɷ�ֹ����Ӧ������e.��Ⱦ������f.�ɷ�ֹ��Ⱦ����������⼸������������ס��ҡ�������װ�õ���ȱ��������������ѡ�������ĿҪ���ѡ�����ڿո��ڡ�
| �ŵ� | ȱ�� | |
| ��װ�� | ||
| ��װ�� | ||
| ��װ�� |
(2)����װ���У�����A��B��������ɣ�����C��D��E��������ɣ�����F��G��������ɣ��������װ���У�ѡȡ��������ɲ��֣���װһ�����Ƶ�ʵ��װ�ã�װ�ø����ֵ�����˳��(�����������ҵķ���)��__________________��
(3)ʵ����������12 mol��L-1��Ũ����100 mL��������MnO2��Ӧ����������Ca(ClO)2�����ʵ�������__________0.15 mol(����ڡ���С�ڡ����ڡ�)����ԭ���ǣ��ٶ�������Ӧ����Ӧ����ģ�������Ӧ������________________________��
ʵ������Ũ���ᡢMnO2������Cl2������Cl2��Ca(OH)2��Ӧ������Ư�ۡ�����֪��Ӧ��
2Cl2+2Ca(OH)2![]() Ca(ClO)2+CaCl2+2H2O
Ca(ClO)2+CaCl2+2H2O
�¶��Ը���������Ӧ��6Cl2+6Ca(OH)2![]() Ca(ClO3)2+5CaCl2+6H2O
Ca(ClO3)2+5CaCl2+6H2O
��������ͬѧ�ֱ���Ƶ�����ʵ��װ�����£�
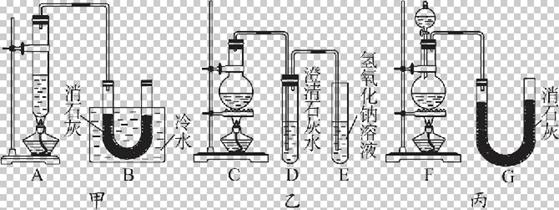
��1����Ӣٲ������Ʒ�Ӧ���ʣ��������Ʒ�Ӧ���ʣ����и���Ӧ�������ܿɷ�ֹ����Ӧ����������Ⱦ�������ɷ�ֹ��Ⱦ������������������ס��ҡ�������װ�õ���ȱ��������������ѡ�������ĿҪ���ѡ�����ڿո��ڡ�
| �ŵ� | ȱ�� | |
| ��װ�� | ||
| ��װ�� | ||
| ��װ�� |
��2������װ���У�����A��B��������ɣ�����C��D��E��������ɣ�����F��G��������ɡ��������װ���У�ѡȡ��������ɲ��֣���װһ�����Ƶ�ʵ��װ�ã�װ�ø����ֵ�����˳�����������ҵķ�����________________________��
��3��ʵ��������12 mol��L-1��Ũ����100 mL��������MnO2��Ӧ����������Ca(ClO)2�����ʵ���_______________0.15 mol(����ڡ���С�ڡ����ڡ�������ԭ����____________���ٶ�������Ӧ����Ӧ����ģ�������Ӧ��������
 Ca(ClO)2
Ca(ClO)2 5CaCl2
5CaCl2