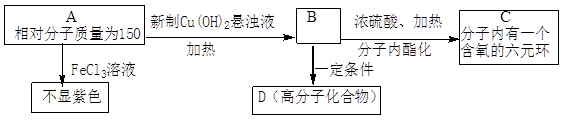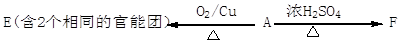��Ŀ����
�������м��ڵĵ�����N1H1����в������������ǰ���ǡ�������Щ��������Ч�ģ��������������ݴ�ѧ�о���Ա��2009��12���о����֣�һЩ�Ǵ���ҩ���簢˾ƥ�ֵ�����ijЩø��ҩ���Ӱ�������Ч�����ñ�ͪΪ��Ҫԭ�Ϻϳɳ�Ч���Ͱ�˾ƥ�ֵ���������ͼ��
�ش��������⣺
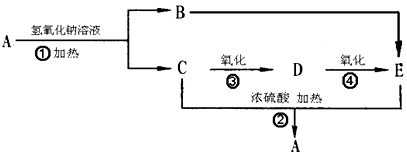
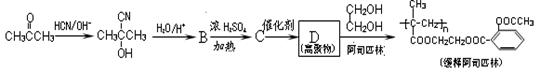
��1�������ϳ�C��·���������л��������壨 ���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�
���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�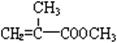 ���仯ѧ����ʽΪ�� ��
���仯ѧ����ʽΪ�� ��
��˸Ľ����ŵ�Ϊ�� ��
��2����˾ƥ�ֵĽṹ��ʽΪ �����������밢˾ƥ�ֻ�Ϊͬ���칹����� ������ĸ����
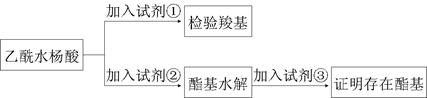
��ij�о���ѧϰС�鿴�����۰�˾ƥ�ֵ�ҩƷ˵���飬Ϊ����֤����ˮ����Ĺ����źͲⶨ��˾ƥ�ֵĴ��ȣ��������ʲ��μӷ�Ӧ��������������ʵ��̽����
��1����˾ƥ����Ч�ɷ����Ȼ������������ŵ���֤
�Լ�����________������Ϊ_________���÷���ͨ����������ˮ����ˮ�����ɵ�____________����ͨ�����������֤�����Ĵ��ڡ�
��2��ȷ��ȡ��˾ƥ��a g�����鲢װ����ƿ����ˮ�ܽ⣬�����Ũ��C1mol/L������������ҺV1mL�����Ƭ�̣�ʹ���ַ�Ӧ����ȴ���÷�̪��ָʾ������C2mol/L����ζ����ζ�ǰ�������ΪV2mL���ζ��յ�ʱ�������ΪV3mL������֪���ζ���Ӧ�ǣ�NaOH+HCl��NaCl+H2O������ˮ�������Է�������Ϊ180��
�ٸ����������ݼ���ð�˾ƥ�ֵĴ���Ϊ ��
�ڵζ��յ��жϡ������� ����������������ƿ��һ�Ű�ֽ���������� ��
�����в���һ���ᵼ�²ⶨ���ƫ�ߵ��� ������ĸ����
E��������Ʒʱ����������ߣ�����Ʒ�����ұ�
F���ü��ȴ����̪��ָʾ��

�ش��������⣺
��1�������ϳ�C��·���������л��������壨
 ���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�
���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�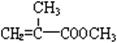 ���仯ѧ����ʽΪ�� ��
���仯ѧ����ʽΪ�� ����˸Ľ����ŵ�Ϊ�� ��
��2����˾ƥ�ֵĽṹ��ʽΪ �����������밢˾ƥ�ֻ�Ϊͬ���칹����� ������ĸ����

��ij�о���ѧϰС�鿴�����۰�˾ƥ�ֵ�ҩƷ˵���飬Ϊ����֤����ˮ����Ĺ����źͲⶨ��˾ƥ�ֵĴ��ȣ��������ʲ��μӷ�Ӧ��������������ʵ��̽����
��1����˾ƥ����Ч�ɷ����Ȼ������������ŵ���֤

�Լ�����________������Ϊ_________���÷���ͨ����������ˮ����ˮ�����ɵ�____________����ͨ�����������֤�����Ĵ��ڡ�
��2��ȷ��ȡ��˾ƥ��a g�����鲢װ����ƿ����ˮ�ܽ⣬�����Ũ��C1mol/L������������ҺV1mL�����Ƭ�̣�ʹ���ַ�Ӧ����ȴ���÷�̪��ָʾ������C2mol/L����ζ����ζ�ǰ�������ΪV2mL���ζ��յ�ʱ�������ΪV3mL������֪���ζ���Ӧ�ǣ�NaOH+HCl��NaCl+H2O������ˮ�������Է�������Ϊ180��
�ٸ����������ݼ���ð�˾ƥ�ֵĴ���Ϊ ��
�ڵζ��յ��жϡ������� ����������������ƿ��һ�Ű�ֽ���������� ��
�����в���һ���ᵼ�²ⶨ���ƫ�ߵ��� ������ĸ����
| A��װ����ǰ���ζ���δ�ñ���Һ��ϴ |
| B���ⶨ�������ʱ��ʼ���Ӷ���������Ӷ��� |
| C����ƿ�ñ�����������Һ��ϴ |
| D���ζ����������ὦ��ƿ�� |
F���ü��ȴ����̪��ָʾ��
��1�� ԭ�������ʴ�100%���ұ���������Ի�������Ⱦ
ԭ�������ʴ�100%���ұ���������Ի�������Ⱦ
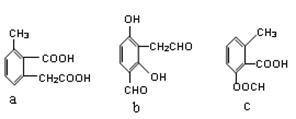
��2��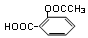 ��bc
��bc
��1��ʯ�ʯ����ɫ�����ǻ�
��2����6(C1V1+C2V2-C2V3)% �ڵ������һ�����ᣬ��Һ�ɺ�ɫǡ�ñ�ɷۺ�ɫ���Ұ���Ӳ���ɫ�����ڹ۲�ζ��յ���Һ��ɫ�仯 ��B
 ԭ�������ʴ�100%���ұ���������Ի�������Ⱦ
ԭ�������ʴ�100%���ұ���������Ի�������Ⱦ ��2��
 ��bc
��bc��1��ʯ�ʯ����ɫ�����ǻ�
��2����6(C1V1+C2V2-C2V3)% �ڵ������һ�����ᣬ��Һ�ɺ�ɫǡ�ñ�ɷۺ�ɫ���Ұ���Ӳ���ɫ�����ڹ۲�ζ��յ���Һ��ɫ�仯 ��B
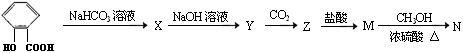
�л��ϳɺ��л��ƶ��ǻ�ѧ�߿������е��ص㣬��֪ʶ����ʱ�µ���Ϣ��ϵ�����ǽ�Щ�����Ҫ���ַ�ʽ������ʵ����ʵ����ѵ㣬Ҳ�Ǹ߿����ȵ㣬��������Ҫ��dz��ߣ���������ǽ��л��ϳɡ������ⶨ����һ�忼���л���ѧ����ʵ���ں�������
�����״����У���1�����ӷ�ָ̪ʾ�������µζ�ԭ����������Ƿ��ƺ������������ᷴӦ��Ȼ���´��⣻��2�����⣨4���С�һ����ʹ�ⶨ���ƫ�ߣ����ѡE����3��������Ӧ���ݼ���ʽ�������������кͷ�Ӧ����ó��෴���ۡ�
��1�������е���Ϣ����д����ѧ����ʽ���˷����ŵ���ԭ�������ʸߣ�û����Ⱦ����2�������������̣��Ƴ�BΪ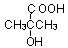 ��B������ȥ��Ӧ��õ�C��C�Ľṹ��ʽΪ
��B������ȥ��Ӧ��õ�C��C�Ľṹ��ʽΪ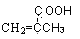 ,�߾���D�Ľṹ��ʽ�ǣ�
,�߾���D�Ľṹ��ʽ�ǣ�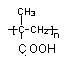 ����D���Ҷ�������˾ƥ�ַ�Ӧ���ɻ��Ͱ�˾ƥ�֣������Ƴ���˾ƥ�ֵĽṹ��ʽΪ
����D���Ҷ�������˾ƥ�ַ�Ӧ���ɻ��Ͱ�˾ƥ�֣������Ƴ���˾ƥ�ֵĽṹ��ʽΪ ��
��
��1���Ȼ��ļ�������������ָʾ������ʯ������DZ��ɫ��������Լ�����ϡ���ᣬͨ����������ˮ����ˮ������ɷ��ǻ��������÷��ǻ�����ɫ��Ӧ����֤���ǻ��Ĵ��ڣ���ӵ�֤�������Ĵ��ڣ���2��������ˮ�������������Ʒ�Ӧ���������������������ᷴӦ�������������Ƶ�����������ˮ���������n(HCl)��(V3-V2)��10-3L��C2mol.L-1��C2��(V3-V2)��10-3mol.�������⣬�ڷ�̪�Լ�ָʾ�£�����ֻ���������Ʒ�Ӧ����������ơ������Ʒ�Ӧ��NaOH+HCl��NaCl+H2O��n(NaOH)��C2(V3-V2)��10-3mol,�밢˾ƥ�ַ�Ӧ���������Ƶ����ʵ���Ϊn(NaOH)��V1��10-3L��C1mol��L-1��C2(V3��V2)��10-3mol��C1V1��10-3mol��C2(V3-V2)��10-3mol��(C1V1+C2V2��C2V3)��10-3mol����������ˮ������Ӧԭ����
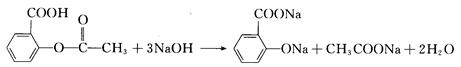
����ˮ��������ʵ���Ϊ(C1V1+C2V2��C2V3)��10-3mol/3��������Ϊm(����ˮ����)��(C1V1+C2V2-C2V3)��10-3mol/3��180g.mol-1��60(C1V1+C2V2-C2V3)��10-3g����(����ˮ����)��60(C1V1+C2V2-C2V3)��10-3g/ag��100%��6(C1V1+C2V2-C2V3)%�����ڼ�Һ�еμӷ�̪�ʺ�ɫ���ζ��յ���ɫ�����һ�����ᣬʹ��Һ�ɺ�ɫ��ɷۺ�ɫ��������ڲ���ɫ����ֽ��������ã����ڹ۲���Һ��ɫ�仯����A��ζ���δ��������ϴ�����µζ��������ƫ��V3ƫ�ⶨ���ƫ�ͣ�B����²ⶨ�������ƫС����V3-V2��ƫС���ⶨ���ƫ��C�������������Һ��ϴ�������������ƫ��V3ƫ�ⶨ���ƫ�ͣ�D������⽦�����²ⶨ�������ƫ�ⶨ���ƫ�͡�E���������Ʒλ�÷ŷ��ˣ�����������ʱ���Գ������û��Ӱ�죻��������ʱ����Ʒ����ƫС���ⶨ��Ʒ����ƫ�ߡ�F����ü�����ָʾ�����ζ��յ�����ԣ��������һ������ƺ������Ʒ�Ӧ������������������ⶨ���ƫ�͡�
�����״����У���1�����ӷ�ָ̪ʾ�������µζ�ԭ����������Ƿ��ƺ������������ᷴӦ��Ȼ���´��⣻��2�����⣨4���С�һ����ʹ�ⶨ���ƫ�ߣ����ѡE����3��������Ӧ���ݼ���ʽ�������������кͷ�Ӧ����ó��෴���ۡ�
��1�������е���Ϣ����д����ѧ����ʽ���˷����ŵ���ԭ�������ʸߣ�û����Ⱦ����2�������������̣��Ƴ�BΪ
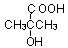 ��B������ȥ��Ӧ��õ�C��C�Ľṹ��ʽΪ
��B������ȥ��Ӧ��õ�C��C�Ľṹ��ʽΪ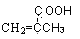 ,�߾���D�Ľṹ��ʽ�ǣ�
,�߾���D�Ľṹ��ʽ�ǣ�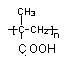 ����D���Ҷ�������˾ƥ�ַ�Ӧ���ɻ��Ͱ�˾ƥ�֣������Ƴ���˾ƥ�ֵĽṹ��ʽΪ
����D���Ҷ�������˾ƥ�ַ�Ӧ���ɻ��Ͱ�˾ƥ�֣������Ƴ���˾ƥ�ֵĽṹ��ʽΪ ��
����1���Ȼ��ļ�������������ָʾ������ʯ������DZ��ɫ��������Լ�����ϡ���ᣬͨ����������ˮ����ˮ������ɷ��ǻ��������÷��ǻ�����ɫ��Ӧ����֤���ǻ��Ĵ��ڣ���ӵ�֤�������Ĵ��ڣ���2��������ˮ�������������Ʒ�Ӧ���������������������ᷴӦ�������������Ƶ�����������ˮ���������n(HCl)��(V3-V2)��10-3L��C2mol.L-1��C2��(V3-V2)��10-3mol.�������⣬�ڷ�̪�Լ�ָʾ�£�����ֻ���������Ʒ�Ӧ����������ơ������Ʒ�Ӧ��NaOH+HCl��NaCl+H2O��n(NaOH)��C2(V3-V2)��10-3mol,�밢˾ƥ�ַ�Ӧ���������Ƶ����ʵ���Ϊn(NaOH)��V1��10-3L��C1mol��L-1��C2(V3��V2)��10-3mol��C1V1��10-3mol��C2(V3-V2)��10-3mol��(C1V1+C2V2��C2V3)��10-3mol����������ˮ������Ӧԭ����

����ˮ��������ʵ���Ϊ(C1V1+C2V2��C2V3)��10-3mol/3��������Ϊm(����ˮ����)��(C1V1+C2V2-C2V3)��10-3mol/3��180g.mol-1��60(C1V1+C2V2-C2V3)��10-3g����(����ˮ����)��60(C1V1+C2V2-C2V3)��10-3g/ag��100%��6(C1V1+C2V2-C2V3)%�����ڼ�Һ�еμӷ�̪�ʺ�ɫ���ζ��յ���ɫ�����һ�����ᣬʹ��Һ�ɺ�ɫ��ɷۺ�ɫ��������ڲ���ɫ����ֽ��������ã����ڹ۲���Һ��ɫ�仯����A��ζ���δ��������ϴ�����µζ��������ƫ��V3ƫ�ⶨ���ƫ�ͣ�B����²ⶨ�������ƫС����V3-V2��ƫС���ⶨ���ƫ��C�������������Һ��ϴ�������������ƫ��V3ƫ�ⶨ���ƫ�ͣ�D������⽦�����²ⶨ�������ƫ�ⶨ���ƫ�͡�E���������Ʒλ�÷ŷ��ˣ�����������ʱ���Գ������û��Ӱ�죻��������ʱ����Ʒ����ƫС���ⶨ��Ʒ����ƫ�ߡ�F����ü�����ָʾ�����ζ��յ�����ԣ��������һ������ƺ������Ʒ�Ӧ������������������ⶨ���ƫ�͡�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
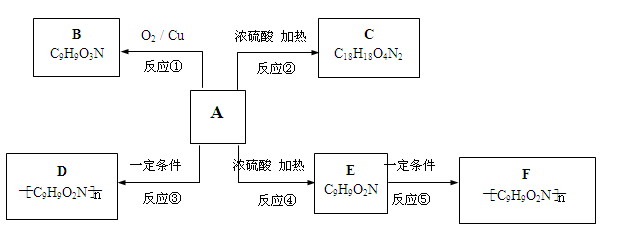
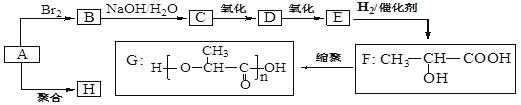
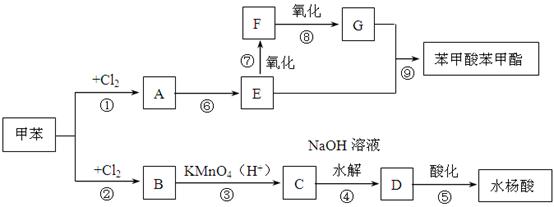
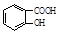 ����ش�
����ش�