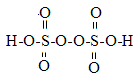��Ŀ����
�������⣨H2O2��O�Ļ��ϼ�Ϊ��1�ۣ��׳�˫��ˮ��ҽ������������ɱ��������������ϴ�˿ڡ��о������漰H2O2�ķ�Ӧ��������и��⣺
A��Ag2O+ H2O2��2Ag+O2 ��+ H2O
B��3H2O2+Cr2(SO4)3+10KOH��2K2CrO4+3K2SO4+8H2O
��1��д��һ��H2O2�����������������ֻ�ԭ�ԵĻ�ѧ��Ӧ����ʽ ��
��2�����������ʣ�H2O2��K2SO4��MnSO4��H2SO4��KMnO4��O2�Ļ�ѧʽ�ֱ����ڿհ״����һ��������ƽ�Ļ�ѧ����ʽ�� + +______ �� + + + H2O
�ٸ÷�Ӧ�еĻ�ԭ���� ��
�ڸ÷�Ӧ�У�������ԭ��Ӧ��Ԫ���� ��
��3�������ԣ�KMnO4_____K2CrO4���������������
A��Ag2O+ H2O2��2Ag+O2 ��+ H2O
B��3H2O2+Cr2(SO4)3+10KOH��2K2CrO4+3K2SO4+8H2O
��1��д��һ��H2O2�����������������ֻ�ԭ�ԵĻ�ѧ��Ӧ����ʽ ��
��2�����������ʣ�H2O2��K2SO4��MnSO4��H2SO4��KMnO4��O2�Ļ�ѧʽ�ֱ����ڿհ״����һ��������ƽ�Ļ�ѧ����ʽ�� + +______ �� + + + H2O
�ٸ÷�Ӧ�еĻ�ԭ���� ��
�ڸ÷�Ӧ�У�������ԭ��Ӧ��Ԫ���� ��
��3�������ԣ�KMnO4_____K2CrO4���������������
��1��2H2O2 2H2O + O2�� ��2��H2O2+KMnO4+H2SO4��MnSO4+O2+K2SO4+ H2O
2H2O + O2�� ��2��H2O2+KMnO4+H2SO4��MnSO4+O2+K2SO4+ H2O
��H2O2 ��Mn ��3����
 2H2O + O2�� ��2��H2O2+KMnO4+H2SO4��MnSO4+O2+K2SO4+ H2O
2H2O + O2�� ��2��H2O2+KMnO4+H2SO4��MnSO4+O2+K2SO4+ H2O��H2O2 ��Mn ��3����
�����������1��H2O2�����������������ֻ�ԭ�ԣ���˵���÷�Ӧһ����˫��ˮ�ķֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ2H2O2
 2H2O + O2����
2H2O + O2������2��������ؾ���ǿ�����ԣ���������ǿ��˫��ˮ�ģ����Ը�������ܰ�˫��ˮ����������������������صĻ�ԭ�����������̣���˸÷�Ӧ�ķ���ʽ���Ա�ʾΪH2O2+KMnO4+H2SO4��MnSO4+O2+K2SO4+ H2O��
��˫��ˮ����Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ������������˫��ˮ�ǻ�ԭ����
�ڸ��������MnԪ�صĻ��ϼ۴ӣ�7�۽��͵���2�ۣ��õ����ӣ�����ԭ�����Ա���ԭ��Ԫ����Mn��
��3���ڷ�Ӧ3H2O2+Cr2(SO4)3+10KOH��2K2CrO4+3K2SO4+8H2O�У�˫��ˮ����������K2CrO4����������������������������ǿ����������Ŀ�֪����������˫��ˮǿ��K2CrO4���ڷ�ӦH2O2+KMnO4+H2SO4��MnSO4+O2+K2SO4+ H2O�У��������������������������ǿ��˫��ˮ�ģ����Ը�����ص�������ǿ��K2CrO4��

��ϰ��ϵ�д�
�����Ŀ
 Si3N4��6CO������������ȷ����(����)
Si3N4��6CO������������ȷ����(����)