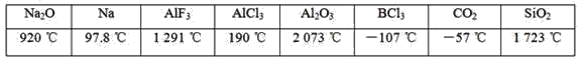
��Ŀ����
����Ŀ�����������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
A. ��֪2SO2(g)��O2(g)![]() 2SO3(g)Ϊ���ȷ�Ӧ����SO2������һ������SO3������
2SO3(g)Ϊ���ȷ�Ӧ����SO2������һ������SO3������
B. ��C(ʯī��s)=C(���ʯ��s)����H>0����ʯī�Ƚ��ʯ�ȶ�
C. ��֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l)����H=��57.4 kJ��mol��1����20.0 g NaOH������ϡ������ȫ�кͣ��ų�28.7 kJ������
D. ��֪2C(s)��2O2(g)=2CO2(g)����H1��2C(s)��O2(g)=2CO(g)����H2������H1>��H2
���𰸡�B
��������
A.��֪2SO2(g)+O2(g)![]() 2SO3(g)Ϊ���ȷ�Ӧ����2molSO2(g)��1molO2(g)������һ������2molSO3(g)����������SO2��������һ������SO3��������A����
2SO3(g)Ϊ���ȷ�Ӧ����2molSO2(g)��1molO2(g)������һ������2molSO3(g)����������SO2��������һ������SO3��������A����
B.��C(ʯī��s)=C(���ʯ��s)����H>0����ʯī���е������ȵ������Ľ��ʯ�������ͣ����ʺ��е�����Խ�ͣ����ʵ��ȶ��Ծ�Խǿ������ʯī�Ƚ��ʯ�ȶ���B��ȷ��
C.��֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l)����H=��57.4 kJ��mol��1��20.0 g NaOH�����ʵ�����0.5mol������NaOH��������ˮ��Ӧ�ų�����������20.0 g NaOH������ϡ������ȫ�кͣ��ų�����������28.7 kJ��C����
D.��������C��ȫȼ�շų����������ڲ���ȫȼ�շų������������ʷ�Ӧ�ų�������Խ�࣬��Ӧ�Ⱦ�ԽС��������H1<��H2��D����
�ʺ���ѡ����B��