��Ŀ����
����Ŀ��2015��10�£��ҹ�����ҩѧ����������������ű������ҩ�������غ�˫���i���ض����2015��ŵ��������ѧ��ҽѧ������Ϊ��λ���ŵ������ѧ���Ļ���Ů��ѧ�ң������磬�ж��й���������(C15H22O5)�Ľṹ��ͼ����ʾ����ش��������⣺
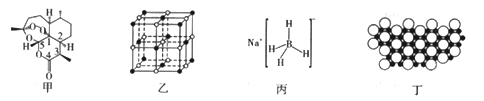
(1)����i���ص�����Ԫ�ص縺���ɴ�С��˳����_________���ڻ�̬Oԭ���У��������________�����������෴�ĵ��ӡ�
(2)���й��������ص�˵����ȷ����_______(�����)��
a.�������мȴ��ڼ��Լ��ִ��ڷǼ��Լ�
b.�������ط����У�����̼ԭ�Ӿ�����ͬһƽ��
c.ͼ�����ֱ�ʶ�����̼ԭ�Ӿ�ֻ�ԦҼ�������ԭ�ӳɼ�
(3)��ȷ�������ؽṹ�Ĺ����У��ɲ������⻯�ƣ�NaBH4����Ϊ��ԭ�������Ʊ�����Ϊ4NaH+ B(OCH3)3![]() NaBH4+3CH3ONa��
NaBH4+3CH3ONa��
��NaHΪ______���壬ͼ����NaH�����ṹ����NaH�������λ����______���������ⳤΪa����Naԭ�Ӽ���С�˼��Ϊ_______��
��B(OCH3)3��B���õ��ӻ�������__________��д��һ����B(OCH3)3������ͬ�ռ乹�͵ķ��ӻ����ӣ�______________________��
��NaBH4�ṹ��ͼ����ʾ���ṹ�д��ڵ���������__________��NaBH4���л���ѧ�е�һ�ֳ��û�ԭ����������ˮ��ˮ������ƫ�����ƺ��������÷�Ӧ�Ļ�ѧ����ʽΪ______________��
(4)��(B)���仯�����ڻ�ѧ������Ҫ�ĵ�λ����ѧ�ҷ�����þ��39kʱ�ʳ����ԣ�����þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ����������С�ͼ���Ǹþ����ۿռ���ȡ���IJ���ԭ����z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬ��ƽ���ϡ�����ͼʾȷ����þ�Ļ�ѧʽΪ_______________��
���𰸡� O>C>H 3 a ���� 6 ![]() sp2 SO3����CO32-�� ���Ӽ�����λ�������ۼ� NaBH4+2H2O=NaBO2+4H2�� MgB2
sp2 SO3����CO32-�� ���Ӽ�����λ�������ۼ� NaBH4+2H2O=NaBO2+4H2�� MgB2
����������1�����⿼��縺�ԵĹ��ɡ�����ԭ���ͺ��ع����������к���C��H��O����Ԫ�أ��縺��Խ�ǽ�����Խǿ����O>C>H����������ԭ����ÿ������������2�����ӣ������������෴�����ع����ǵ������ȵ���ռ��һ�������������������ͬ�������Ԫ����3�����������෴�ĵ��ӣ���2�����黯ѧ�������͡��ӻ����ͣ�a�����������صĽṹ��ʽ��C��H��O��H��C��O֮���Ǽ��Լ���O��O֮���ǷǼ��Լ�����a��ȷ��b�����������е�̼ԭ��ʱap3�ӻ����������̼ԭ�Ӳ����棬��b����C��4��̼ԭ�����Цм�����c����3�����龧���ļ��㡢�ռ乹�ͣ���NaH�����ӻ�����������Ӿ��壻���Ӿ������λ��Ϊһ��������Χ����������ӵĸ���������λ��Ϊ6��Na��������ĺ˼������Խ��ߵ�1/2����Ϊ![]() ���ڸ��ݻ�ѧʽB(OCH3)3��Bԭ����3����ԭ���γɦҼ������µ��Ӷԣ����B���ӻ�����Ϊsp2��B(OCH3)3�Ŀռ乹��Ϊƽ�������Σ���B(OCH3)3������ͬ�ռ乹��Ϊ���ӻ�������SO3��CO32������Bԭ���������3�����ӣ�NaBH4�������ӻ�����������Ӽ���B��3��H֮���γɹ��ۼ���B��H��֮���γ���λ����������Ŀ��Ϣ��������Ӧ�ķ���ʽΪNaBH4��2H2O=NaBO2��4H2������4�����黯ѧʽ������ͶӰ��֪��1��Bԭ����3��Mgԭ�ӹ��ã��������һ��Mgԭ�ӵ�Bԭ��Ϊ1/3��1��Mgԭ��Ϊ6��Bԭ�ӹ��ã��������1��Bԭ�ӵ�Mgԭ��Ϊ1/6�����Bԭ�Ӻ�Mgԭ�Ӹ�����Ϊ��1/3��1/6=2��1������ѧʽΪMgB2��
���ڸ��ݻ�ѧʽB(OCH3)3��Bԭ����3����ԭ���γɦҼ������µ��Ӷԣ����B���ӻ�����Ϊsp2��B(OCH3)3�Ŀռ乹��Ϊƽ�������Σ���B(OCH3)3������ͬ�ռ乹��Ϊ���ӻ�������SO3��CO32������Bԭ���������3�����ӣ�NaBH4�������ӻ�����������Ӽ���B��3��H֮���γɹ��ۼ���B��H��֮���γ���λ����������Ŀ��Ϣ��������Ӧ�ķ���ʽΪNaBH4��2H2O=NaBO2��4H2������4�����黯ѧʽ������ͶӰ��֪��1��Bԭ����3��Mgԭ�ӹ��ã��������һ��Mgԭ�ӵ�Bԭ��Ϊ1/3��1��Mgԭ��Ϊ6��Bԭ�ӹ��ã��������1��Bԭ�ӵ�Mgԭ��Ϊ1/6�����Bԭ�Ӻ�Mgԭ�Ӹ�����Ϊ��1/3��1/6=2��1������ѧʽΪMgB2��

����Ŀ��һ���¶��£����������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g)![]() CH3OCH3(g)��H2O(g) ����˵����ȷ����
CH3OCH3(g)��H2O(g) ����˵����ȷ����
��� | �¶� | ��ʼ���ʵ���(mol) | ƽ�����ʵ���(mol) | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | 387�� | 0.20 | 0.080 | 0.080 |
�� | 387�� | 0.40 | ||
�� | 207�� | 0.20 | 0.090 | 0.090 |
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. �ﵽƽ��ʱ���������е�CH3OH����������������е�С
C. �������з�Ӧ����ƽ������ʱ����������еij�
D. ����ʼʱ���������г���CH3OH 0.15 mol��CH3OCH3 0.15 mol��H2O 0.10 mol����Ӧ��������Ӧ�������