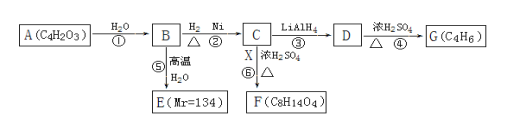
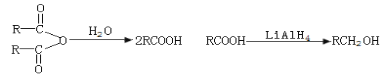
��Ŀ����
����Ŀ��ij���Ͻ��к�����������þ��ͭ������Ϊ�˲ⶨ�úϽ������ĺ����������������ʵ�飺
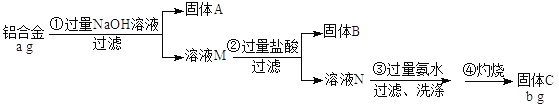
��֪��Si+2NaOH+ H2O =Na2SiO3+2H2����H2SiO3�Dz�����ˮ�����ᡣ
��1������A�ijɷ��ǣ�____________����ҺM�е��������У�____________________
��2�������٢ڢ��У�����ʱ�������������� ���������У��õ������������żܡ��ƾ����Լ�
��3�������������ɳ��������ӷ���ʽΪ��____________________________
��4���ڿ����н�NaOH��Һ����FeCl2��Һ�У��۲쵽�������� ,��Ӧ�Ļ�ѧ����ʽ��
��5������Ʒ������������������_____________________����a��b��ʾ����
��6���������еij�����������ˮϴ������δϴ����ʹ�ⶨ���___________����ƫ���ƫС����
���𰸡���1��Mg��Cu��Fe(2��)OH����AlO2����SiO32��(2��)
��2������(2��)�����������ǡ�������ǯ��(2��)
��3��Al3��+3NH3��H2O��Al(OH)3��+3NH4��(2��)
��4�����ְ�ɫ������Ѹ����Ϊ����ɫ�������ձ�Ϊ���ɫ������(2��)
FeCl2+2NaOH=Fe(OH)2��+2NaCl(2��)
4Fe(OH)2+O2+2H2O=4Fe(OH)3(2��)
��5��![]() (2��)��6��ƫ��(2��)
(2��)��6��ƫ��(2��)
��������
�����������1����������þ��ͭ�������������Ʒ�Ӧ��ֻ�������裬����ƫ�����ơ������ƣ�����������ʣ�࣬�����A�ijɷ���Mg��Cu��Fe����ҺM�е��������У�OH����AlO2����SiO32����
��2�������٢ڢ��У�����ʱ�������������������������������գ��õ������������żܡ��ƾ����Լ������������ǡ�����ǯ��
��3�������ڹ�����ˮ���γɳ��������ɳ��������ӷ���ʽΪAl3��+ 3NH3��H2O��Al(OH)3��+ 3NH4����
��4���ڿ����н�NaOH��Һ����FeCl2��Һ���������������������������������ȶ����ܱ������е������������۲쵽����������ɫ��״����Ѹ��ת���ɻ���ɫ�������ת���ɺ��ɫ��������Ӧ�Ļ�ѧ����ʽ��FeCl2+2NaOH=Fe(OH)2��+2NaCl��4Fe(OH)2+O2+2 H2O = 4Fe(OH)3��
��5���������̸�����ԭ���غ�ù�ϵʽΪ2Al��Al2O3������Ʒ��������������Ϊ{[(b/102)��2��27]��a}��100%��![]() ��
��
��6���������еij���δ������ˮϴ�ӣ��������������������ⶨ���ƫ�ߡ�