��Ŀ����
�����£�N2H4���ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�á��ش��������⣺
��1����֪��N2(g)+3H2(g)  2NH3(g) ��H��-92.4kJ��mol-1
2NH3(g) ��H��-92.4kJ��mol-1
�ں��¡����ݵ��ܱ������У��ϳɰ���Ӧ�ĸ�����Ũ�ȵı仯������ͼ��ʾ��
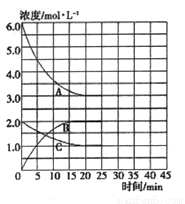
�� �����ڸ��¶��·�Ӧ2NH3(g)  N2(g)+3H2(g)��ƽ�ⳣ��K=________��
N2(g)+3H2(g)��ƽ�ⳣ��K=________��
�� �ڵ�25minĩ�����������������䣬�����¶Ƚ��ͣ��ڵ�35minĩ�ٴδﵽƽ�⡣��ƽ���ƶ�������N2Ũ�ȱ仯��0.5mol/L������ͼ�л���25-40minNH3Ũ�ȱ仯���ߡ�________
�� ��֪��2N2(g)+6H2O(l)  4NH3(g)+3O2��g����H=+1530.0KJ/mol����������ֵΪ_____��
4NH3(g)+3O2��g����H=+1530.0KJ/mol����������ֵΪ_____��
��2���� N2H4��һ�ָ���ȼ�Ͼ��л�ԭ�ԣ�ͨ����NaClO�����NH3��Ӧ�Ƶã������Ϊʲô�ù���������Ӧ��ԭ��__________
�� ��NaClO��NH3 ��N2H4�ķ�Ӧ���൱���ӵģ���Ҫ��Ϊ������
��֪��һ����NH3+ClO-=OH-+NH2Cl
��д���ڶ������ӷ���ʽ��__________________
�� N2H4������ˮ�����백�����Ƶ������֪�䳣���µ��볣��K1=1.0��10-6�������£���0.2 mol/L N2H4��H2O��0.lmol/L������������ϣ���������仯�������ʱ��Һ��PH����________������N2H4�Ķ������룩��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�CaS�������Ʊ������ᡢ������ë����ɱ���������ȣ�������ҽҩ��ҵ���ؽ��������������С�ij��ѧ̽��С����ʵ���������÷�Ӧ�� CaSO4+C CaS+CaO+SO2��+CO��+CO2���Ʊ��Ʋ���������ѡ�õ�װ�����¡��ش��������⣺
CaS+CaO+SO2��+CO��+CO2���Ʊ��Ʋ���������ѡ�õ�װ�����¡��ش��������⣺
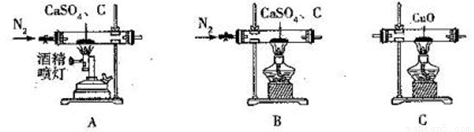

��֪:��C��E ~ H�е��Լ���Ϊ������
������KMnO4��Һ��������ʱ����Ԫ�ر���ԭ��Mn2+
��CaS��H2O��Ӧ����Ca(OH)2��H2S��
(1)ʵ��1����ֻ�������������Ƿ���CO���ɲ��õ�װ�����Ϊ______(��������������ĸ��ϣ����������д���CO��������________________________��
(2)ʵ��2����ֻ�������������Ƿ���CO2������õ����װ�����Ϊ______(��������������ĸ��ϣ�����ȥSO2�����ӷ���ʽΪ__________________________��
(3)ʵ��3����ͬʱ����CO2��SO2�����õ�װ�����Ϊ_______ (��������������ĸ��ϣ���
(4)���Ʊ���Ӧ��������ȫ��ͬ��������������ȫ��ͨ������ʵ��װ���У��õ����й��������±�:
ʵ��ǰ | ʵ��� | |
ʵ��1 | װ��CӲ�ʲ�������ͬCuO����Ϊ26.80 g | װ��CӲ�ʲ�������ͬ��Ӧ���������Ϊ26.64 g |
ʵ��2 | װ��G��ͬ��Һ����Ϊ187.50g | װ��G��ͬ��Ӧ����Һ������������Ϊ188.38 g |
ʵ��3 | װ��D��H��ͬ��Һ������Ϊ373.60g | װ��D��H��ͬ��Һ������Ϊ374.24 g |
д��CaSO4�ͽ�̿�ڸ��������·�Ӧ����CaS�Ļ�ѧ����ʽ��_______________��
(5)Ϊ��֤���ƵĻ�ѧʽ�������ʵ�飺��һ������(m1)����Ʒ���������ı���Na2CO3��Һ�У�ͨ��____�������Ƶù�������Ϊm2�����ʾ����ɵļ���ʽΪn(Ca):n(S)=__________(�ú�m1 ��m2�Ĵ���ʽ��ʾ)��
ij��ҵ��ˮ�н����±������е�5�֣�
������ | K+ Cu2+ Fe3+ Ca2+ Fe2+ |
������ | Cl- CO32- NO3- SO42- SiO32- |
ijͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��ȡ��ˮ���������������ᣬ�ް�ɫ��������,��������ʹ����ʯ��ˮ����ǵ���ɫ��ζ����
����������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
�����ƶϲ���ȷ����
A. ��Һ��һ�����е���������K+ �� Cl- ��CO32- ��NO3- �� SO42-
B. ���м�������������ɫ����ĵ����ӷ���ʽ��CO32-+2H+=CO2��+H2O
C. ԭ��Һ�е�K+ �� Cl- ��NO3- ���������ȷ��
D. ���в�����ɫ���������ӷ���ʽ��Ba2++SO42-=BaSO4��


 ����ԭ�� D. ����֪��Ϣ�ɵã�
����ԭ�� D. ����֪��Ϣ�ɵã� [
[
