��Ŀ����
ij�ѧ��ȤС��������ʵ�飬����ش�������⣺
����ͬѧ���ý����ƺͿ����Ʊ����Ƚϸߵ�Na2O2(�����ǿ�����N2)�������õ�װ�����¡��ش��������⣺
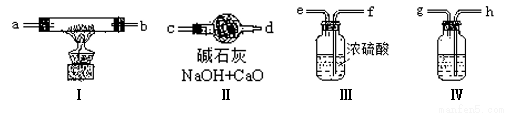
(1)װ�â���ʢ�ŵ�ҩƷ�� ����û�и�װ�ÿ��ܵ������ɵ�Na2O2�к��� ���䷴Ӧ����ʽΪ ��
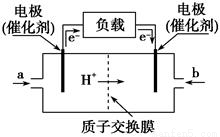
(2)���涨�����������������ң��������ӿڵı����ĸ(a��b����)˳���ǣ�
�������� �� �� �� �� �� �� ��
(3)װ�õĢ������� ��
������������ͼ��ʾʵ��װ�ý���ijЩ������Ʊ������ʵ�����(ͼ�мг�װ����ʡ��)��
(4)Ϊ��֤��������ʹ����ĺ첼����ɫ���ú첼����B�У���A-C-B-D���ӳ�ʵ��װ����ϵ��A��ƿ���Լ�ѡ�ø�����ؾ��壬���Һ©���е�Һ���� ��A�з����Ļ�ѧ����ʽ�� ��C���Լ��� ��D�������� ��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 5N2(g)+6H2O(g) ��H1
5N2(g)+6H2O(g) ��H1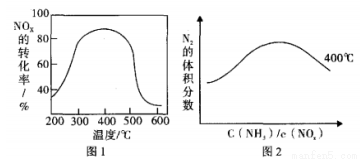
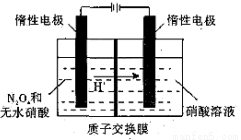
 4NO(g)+6H2O(g)��H=-905.5kJ•mol-1
4NO(g)+6H2O(g)��H=-905.5kJ•mol-1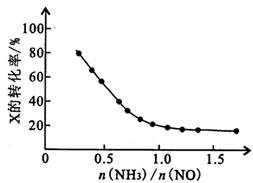

 ����Cl����HCO3�� B��K����Fe3����NO3����SO42��
����Cl����HCO3�� B��K����Fe3����NO3����SO42��