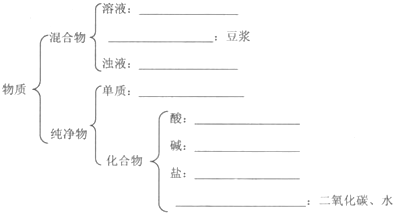
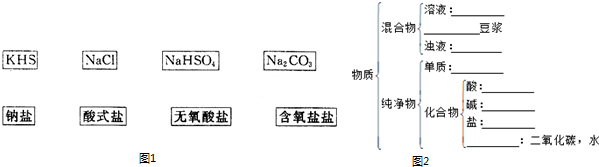
��Ŀ����
�����������ʣ�
��H2S��
��NH3��
��H2SO4��
��NaHCO3��
��CH3COOH��
��KNO3��Һ��
�����ᡢ
��ƾ���
���Ԫ��H2A�ȣ�
��ش���������(���������)��
(1)����һ������������ʵ���________������ȷ������________�������һ��ʵ����֤������ǿ����ʻ���������ʣ����ʵ��ͽ�����________��
(2)H2S��ˮ�еĵ��뷽��ʽΪ________��NaHCO3��ˮ�еĵ��뷽��ʽΪ________����֪H2A�ĵ��뷽��ʽΪ��H2A![]() H+��HA����HA��
H+��HA����HA��![]() H+��A2�������ж�H2A��________�����(�ǿ��������)��
H+��A2�������ж�H2A��________�����(�ǿ��������)��
(3)2 mol/L�������2 mol/L�Ĵ����100 ml���ֱ��������Zn��Ӧ������H2�������V(����)________V(����)(�������)��������Ũ����ȵ�����ʹ����100 ml���ֱ��������Zn��Ӧ������H2�����V(����)________V(����)(�������)��������________��
(4)��֪CH3COO����H+![]() CH3COOH����Ҫʹƽ�������ƶ���������Ũ������Ӧ��ȡ�Ĵ�ʩ��________
CH3COOH����Ҫʹƽ�������ƶ���������Ũ������Ӧ��ȡ�Ĵ�ʩ��________
A����NaOH
B��������
C����ˮ
D�������¶�
��֪CH3COOH���ܼ�A�п���ȫ�����룬�β��ܽ���A�ܼ�����CH3COOH���ܼ�A�еĵ��뷽��ʽΪ________��CH3COOH��Na2CO3���ܼ�A������CO2��Ӧ�����ӷ���ʽ��________��