��Ŀ����
(15��)�Ķ��������ϣ��ݴ��������Ҫ��
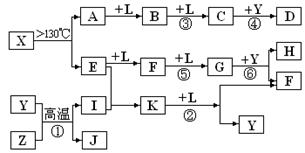
������Ԫ��A��B��C��D��E��F�����ǵ�ԭ������������������B��CΪͬһ���ڣ�D��E��FΪͬһ���ڣ�A��D��C��F�ֱ�Ϊͬһ���壻CԪ��ԭ�ӵ������������Ǵ�����������3����D����������ԭ�Ӱ뾶����Ԫ��(��ϡ��������)��E�Ƿǽ���Ԫ�أ��䵥���ڵ��ӹ�ҵ������Ҫ�ô���
��AԪ����̼Ԫ�ذ�������1��3�����γɻ�����X����C2��X��23.4gD2C2�����ܱ������У��õ������ѧ��Ӧ����Ӧ�����������ڵ���ѹΪ��(250��)��������������ˮ�У��������������C2��X�����ʵ���֮��Ϊ �������䷴Ӧ����ʽ����������Ӧ����4mol����ת�ƣ�������C2�����ʵ���Ϊ mol��
�ƹ�ҵ����������E�Ļ�ѧ����ʽΪ�� ��ָ��E����������ͨѶ�е�һ����; ��
��һ�������£�A2������B2�����ַ�Ӧ������6.8g�ռ乹�������ε����壬�ų�18.44kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪΪ�� ��A��B��ԭ�Ӹ�����1��2���γ����ԭ������Ϊ32�Ļ����д���û��������������ᷴӦ�����ӷ���ʽ ��
��A2��C2��KOH��Ũ��Һ�п����γ�ԭ��ء������PtΪ�缫���ڵ�ص������ֱ�ͨ��A2��C2����ͨ���A2һ���ĵ缫��ӦʽΪ�� ��
����10L���ܱ������У�ͨ��2molFC2��3molC2���壬һ�������·�Ӧ������FC3���壬����Ӧ�ﵽƽ��ʱ��C2��Ũ��Ϊ0.21mol/L����ƽ��ʱFC2��ת����Ϊ ��
������Ԫ��A��B��C��D��E��F�����ǵ�ԭ������������������B��CΪͬһ���ڣ�D��E��FΪͬһ���ڣ�A��D��C��F�ֱ�Ϊͬһ���壻CԪ��ԭ�ӵ������������Ǵ�����������3����D����������ԭ�Ӱ뾶����Ԫ��(��ϡ��������)��E�Ƿǽ���Ԫ�أ��䵥���ڵ��ӹ�ҵ������Ҫ�ô���
��AԪ����̼Ԫ�ذ�������1��3�����γɻ�����X����C2��X��23.4gD2C2�����ܱ������У��õ������ѧ��Ӧ����Ӧ�����������ڵ���ѹΪ��(250��)��������������ˮ�У��������������C2��X�����ʵ���֮��Ϊ �������䷴Ӧ����ʽ����������Ӧ����4mol����ת�ƣ�������C2�����ʵ���Ϊ mol��
�ƹ�ҵ����������E�Ļ�ѧ����ʽΪ�� ��ָ��E����������ͨѶ�е�һ����; ��
��һ�������£�A2������B2�����ַ�Ӧ������6.8g�ռ乹�������ε����壬�ų�18.44kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪΪ�� ��A��B��ԭ�Ӹ�����1��2���γ����ԭ������Ϊ32�Ļ����д���û��������������ᷴӦ�����ӷ���ʽ ��
��A2��C2��KOH��Ũ��Һ�п����γ�ԭ��ء������PtΪ�缫���ڵ�ص������ֱ�ͨ��A2��C2����ͨ���A2һ���ĵ缫��ӦʽΪ�� ��
����10L���ܱ������У�ͨ��2molFC2��3molC2���壬һ�������·�Ӧ������FC3���壬����Ӧ�ﵽƽ��ʱ��C2��Ũ��Ϊ0.21mol/L����ƽ��ʱFC2��ת����Ϊ ��
(15��)
 (1)1:2(2��) 0.25(2��)
(1)1:2(2��) 0.25(2��)
(2)SiO2+2C Si+2CO(2��) �����ά(1��)(3)N2(g)+3H2(g) 2NH3(g)����H=��92.2kJ/mol(2��)
2NH3(g)����H=��92.2kJ/mol(2��)
N2H4+2H��=N2H62��(2��)
(4)H2��2e��+2OH��=2H2O(2��)
(5)90%
 (1)1:2(2��) 0.25(2��)
(1)1:2(2��) 0.25(2��)
|
 2NH3(g)����H=��92.2kJ/mol(2��)
2NH3(g)����H=��92.2kJ/mol(2��) N2H4+2H��=N2H62��(2��)
(4)H2��2e��+2OH��=2H2O(2��)
(5)90%
���Ƴ�A��B��C��D��E��F�ֱ�Ϊ��H��N��O��Na��Si��S��XΪ���飬������ӦΪ��2CH4+O2+6Na2O2=2Na2CO3+8NaOH,�ʣ�1���������3��������5����

��ϰ��ϵ�д�
�����Ŀ

 ����������������������������������������
����������������������������������������