��Ŀ����
����Ŀ����Ի�ѧ��Ӧ�е������仯�������������
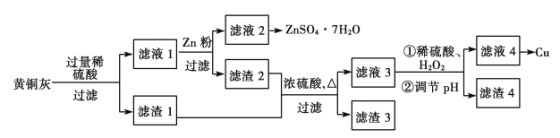
��1���ⶨϡ�����ϡ���������к������к���Ϊ57.3 kJ.mor1����ʵ��װ�D��ͼ��ʾ��
ij��ȤС���ʵ����ֵ���С��57.3 kJ/mol��ԭ�������________������ĸ����
A��ʵ��װ�D���¡�����Ч����
B����ȡ���Һ������¶ȼ�Ϊ�յ��¶�
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ���Ⱥ�δϴ�ӣ�ֱ�ӲⶨH2SO4 ��Һ���¶�
��2����������������ȡ��ҵԭ����������֪��
A��CH3COOH��l��+2O2��g��=2CO2��g��+2H2O��l�� ��H=��870.3kJ��mo1
B��C��s��+O2��g��=CO2��g�� ��H=��393.5kJ��mo1
C��H2��g��+1/2O2��g��=H2O��l�� ��H=��285.8kJ��mo1
����ͬ������CH3COOH��C��H2��ȫȼ��ʱ���ų�����������___________��
������������Ϣ����������Ӧ��2C��s��+2H2��g��+O2��g��=CH3COOH��l�� ��H= kJ/mol ��
��3����Cl2����ijЩ�����л���ʱ�����������HC1�����÷�ӦA����ʵ������ѭ�����á�
��Ӧ A: 4HCl��O2![]() 2Cl2��2H2O
2Cl2��2H2O
��1����֪����.��ӦA�У�4 molHCl���������ų�115.6 kJ��������
![]()
��.![]()
��д���������·�ӦA���Ȼ�ѧ����ʽ��________________��
�Ͽ�1 mol H��O����Ͽ�1 mol H��Cl�������������ԼΪ________kJ��
���𰸡���1��acd��3������
��2����H2��2������-488.3kJ/mol��2������
��3����4HCl��g����O2��g��![]() 2Cl2��g����2H2O��g�� ��H����115.6kJ/mol��31.9
2Cl2��g����2H2O��g�� ��H����115.6kJ/mol��31.9
��������
�����������1��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��b����Ӧ������¶ȼ�Ϊ�յ��¶ȣ���b����c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��������������ɢʧ���к��ȵ���ֵƫС����c��ȷ��d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ������¶ȼ��ϻ����������ƣ��������������ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ��к��ȵ���ֵƫС����d��ȷ;�ʴ�Ϊacd��
��2����1gCH3COOH��C��H2��ȫȼ��ʱ�ų��������ֱ�Ϊ��870.3kJ/60g��393.5kJ/12g��285.8kJ/2g��������ͬ������CH3COOH��C��H2��ȫȼ��ʱ���ų�����������H2��
�����ø�˹���ɣ�������2+����2-���ɵ�2C��s��+2H2��g��+O2��g���TCH3COOH��l��������H4=2����-393.5kJ/mol��+2����-285.8kJ/mol��-��-870.3kJ/mol��=-488.3kJ/mol��
��3�������ݷ�ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ���Ȼ�ѧ����ʽΪ��4HCl��g��+O2��g��2Cl2��g��+2H2O��g����H=-115.6 KJ/mol�����ʱ�=��Ӧ��ϼ���������-�������γɻ�ѧ���ų�������4HCl��g��+O2��g��2Cl2��g��+2H2O��g����H=-115.6 KJ/mol��4��E��H-Cl��+498-[243��2+4��E��H-O��]=-115.6���õ�4��E��H-O��-4��E��H-Cl��=498-486+115.6=127.6��E��H-O��-E��H-Cl��=31.9��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ(ʡ�Լгֺ;���װ��)�����ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����( )
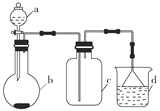
ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | Ũ��ˮ | CaO | NH3 | H2O |
B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
C | Ũ���� | Cu | NO2 | H2O |
D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
A. A B. B C. C D. D