��Ŀ����
��һ����Һ�ף���ȷ���Ƿ����������ӣ�Na+��Mg2+��Al3+��Ba2+��SO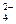 ��Cl����I����HCO
��Cl����I����HCO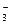 ����ȡ����Һʵ�����£�
����ȡ����Һʵ�����£�
��1����ʵ�鲽����жϳ���Һ�п϶������ڵ������� ����˵����һʵ����ʵ�����ӷ���ʽ��
��2��ʵ�鲽��۵�ʵ����˵����Һ�в����� ����
��3����ȷ���������п϶����ڵ��� ��
��4��Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ����
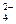 ��Cl����I����HCO
��Cl����I����HCO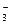 ����ȡ����Һʵ�����£�
����ȡ����Һʵ�����£�| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���μӼ��μ��� | ��Һ���ɫ |
| ��ȡ��������Һ���μ�Ba��NO3��2��Һ | ��Һ�����Ա仯 |
| ��ȡ����ʵ������Һ���μ�AgNO3��Һ | �а�ɫ�������ɣ��Ҳ�����HNO3 |
| ��ȡ��������Һ���μ�NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
��2��ʵ�鲽��۵�ʵ����˵����Һ�в����� ����
��3����ȷ���������п϶����ڵ��� ��
��4��Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ����
| A������ | B������ | C����ɫ��Ӧ | D������ |
��1��HCO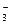 ��2�֣� H+ + HCO
��2�֣� H+ + HCO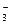 = CO2��+ H2O ��3�֣�
= CO2��+ H2O ��3�֣�
��2��I-, (2��)
��3��Mg2+��Al3+��Cl- (6��ÿ������2�֣�����������1�ֿ�2�֣����Ƹ���)
��4��C��3�֣�
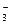 ��2�֣� H+ + HCO
��2�֣� H+ + HCO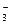 = CO2��+ H2O ��3�֣�
= CO2��+ H2O ��3�֣���2��I-, (2��)
��3��Mg2+��Al3+��Cl- (6��ÿ������2�֣�����������1�ֿ�2�֣����Ƹ���)
��4��C��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 O�����������Դ�����Һ�ǵ����
O�����������Դ�����Һ�ǵ����