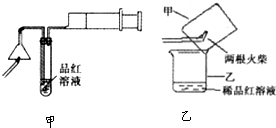
��Ŀ����
ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ������ʵ��ѡ��ϸͭ˿��98.3%H2SO4��Ʒ����Һ������ʯ��ˮ��CC14��NaOH��Һ��ҩƷ��ͭ˿����������״��һ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
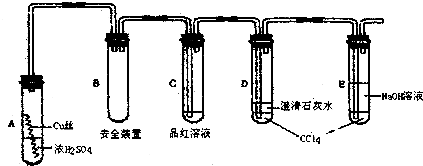
�����Ǹ�ѧϰС�鲿�ֽ�����¼������̽��ʵ��ļ�¼

�����������ϻش��������⣺
��1��A�Թ��Ϸ��ij����ܵ�������
��2�����ȹ����У��۲쵽A�Թ��г��ִ�����ɫ��������������������Թ��ϲ��ڱ���������ɫ�������ʣ��ڳ�������Ũ���ᣨ���ڣ�ʱ������ɫ������������������ʧ��д������ɫ������ʧ�Ļ�ѧ��Ӧ����ʽ��
��3����A�Թ��е�ŨH2SO4��ͭ˿���м��ȣ��ܿ췢��C�Թ���Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ������ѧ��1����������SO2�ܽ�Ƚϴ�������Ca��HSO4��2��Ե�ʣ��������ʵ����֤ѧ��1�IJ���
��4�����������о��������ѧ֪ʶ������ΪҺ���·�ͭ˿����ĺ�ɫ���ʳɷ���
��5��ѧ��2����Ӧ��ķ�ӦҺϡ�ͺ���м��飬������Һ�����ԣ��Դˣ�ѧ��2����IJ���ģ�ͭ��Ũ���ᷴӦ���ɵ�����ͭˮ����Һ�����ԣ�
��IJ����ǣ�
�������һ����ʵ������֤��IJ��룺
��������1�������ܳ��˵�������֮�⣬���������������������������������ǿ��ķ�Ӧʮ�־��ң�Ҫע���ֹ��������ķ�����
��2�����ڼ���ʱ�ܺ�Ũ���ᷴӦ���ɶ��������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��3����ʽ�κ�ǿ���е���������Ӧ�ܵõ����Σ�����ʹ������������Һ���м��飻
��4������ͭԪ�صij�����ɫ������CuO��CuS��Cu2S�����ʵ�����ش�
��5������ʵ��ʵ��������ж���Һ�����ԵĿ���ԭ����������֤��
��2�����ڼ���ʱ�ܺ�Ũ���ᷴӦ���ɶ��������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��3����ʽ�κ�ǿ���е���������Ӧ�ܵõ����Σ�����ʹ������������Һ���м��飻
��4������ͭԪ�صij�����ɫ������CuO��CuS��Cu2S�����ʵ�����ش�
��5������ʵ��ʵ��������ж���Һ�����ԵĿ���ԭ����������֤��
����⣺��1������ʵ��װ�ã������ܵ�����һ�����ܵ������������ڵ�����ʱ��һ���ͻ����������������������ǿ��ķ�Ӧʮ�־��ң����Բ������Ȼ�̼���������ﵽ��ֹ��������ķ�����Ŀ�ģ�
�ʴ�Ϊ����������������������ֹ������
��2��Ũ�������ǿ�����ԣ����ڼ���ʱ�ܺ�Ũ���ᷴӦ����Ӧ�Ļ�ѧ����ʽΪS+2H2SO4��Ũ��
3SO2��+2H2O��
�ʴ�Ϊ��S+2H2SO4��Ũ��
3SO2��+2H2O��
��3��Ca��HSO4��2�ܺ�ǿ�Ӧ�����ܽ�Ƚ�С��������ƣ����Լ�������������Һ���۲��Ƿ��г������ɣ�
�ʴ�Ϊ��ȡ���������м�����������Һ���۲��Ƿ��г������ɣ�
��4������ͭԪ�صĺ�ɫ�����У�CuO��CuS��Cu2S������������Ϣ��֪������Һ���·�ͭ˿����ĺ�ɫ���ʳɷ�ΪCuO��CuS��Cu2S��
�ʴ�Ϊ��CuO��CuS��Cu2S��
��5����Һ�����ԵĿ���ԭ����������������Լ����������������Һ�������ӵĴ��ڣ�����Ϊ��ȡϡ�ͺ����Һ�������Թ��У��������������ۣ�������ɫ��ζ�����������֤�����������
�ʴ�Ϊ��ͭ��Ũ���ᷴӦ��ʵ�����������ʹ��Һ�����ԣ�ȡϡ�ͺ����Һ�������Թ��У��������������ۣ�������ɫ��ζ�����������֤�����������������Ҳ��������
�ʴ�Ϊ����������������������ֹ������
��2��Ũ�������ǿ�����ԣ����ڼ���ʱ�ܺ�Ũ���ᷴӦ����Ӧ�Ļ�ѧ����ʽΪS+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��S+2H2SO4��Ũ��
| ||
��3��Ca��HSO4��2�ܺ�ǿ�Ӧ�����ܽ�Ƚ�С��������ƣ����Լ�������������Һ���۲��Ƿ��г������ɣ�
�ʴ�Ϊ��ȡ���������м�����������Һ���۲��Ƿ��г������ɣ�
��4������ͭԪ�صĺ�ɫ�����У�CuO��CuS��Cu2S������������Ϣ��֪������Һ���·�ͭ˿����ĺ�ɫ���ʳɷ�ΪCuO��CuS��Cu2S��
�ʴ�Ϊ��CuO��CuS��Cu2S��
��5����Һ�����ԵĿ���ԭ����������������Լ����������������Һ�������ӵĴ��ڣ�����Ϊ��ȡϡ�ͺ����Һ�������Թ��У��������������ۣ�������ɫ��ζ�����������֤�����������
�ʴ�Ϊ��ͭ��Ũ���ᷴӦ��ʵ�����������ʹ��Һ�����ԣ�ȡϡ�ͺ����Һ�������Թ��У��������������ۣ�������ɫ��ζ�����������֤�����������������Ҳ��������
���������⿼����Ũ��������ʣ���Ŀ�Ѷ��еȣ�ע������Ũ������еĻ�ѧ���ʣ���ȷ����ʵ�鷽����Ƶķ�������ȷ����������Ϣ�ǽ�����ݣ�����������ѧ���ķ���������������

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
�����Ŀ
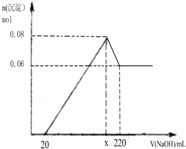
ij�о���ѧϰС����ݺ�°��Ƽԭ�����ο��±��й������ܽ�ȣ�g/100gˮ�����ݣ��Ա���NaCl��Һ����ϸ��NH4HCO3Ϊԭ�ϣ����ʵ�龭������Ӧ�Ʊ����
�����й�˵���У���ȷ���ǣ�������
| ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | 35�����Ϸֽ� | |||
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
| A����һ��������Ӧ�Ļ�������Ϊ���Ϸ�Ӧ���ֽⷴӦ |
| B����һ��������Ӧ֮�����Ҫʵ������ǹ��ˡ�ϴ�� |
| C����һ����Ӧ�����¶ȸ���30��Ŀ������߷�Ӧ���� |
| D����ӦҺ�����ᴦ����ʹNaClѭ��ʹ�ò�����NH4Cl |
 ��һ�ֺ�������Ҫ�ɷ���Fe2O3���������ʲ�����ˮ���ᣮij�о���ѧϰС���ͬѧ������һС����Ʒ������������ʵ�飮
��һ�ֺ�������Ҫ�ɷ���Fe2O3���������ʲ�����ˮ���ᣮij�о���ѧϰС���ͬѧ������һС����Ʒ������������ʵ�飮