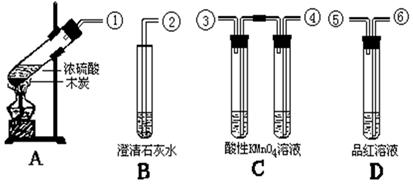
��Ŀ����
(10��) ��ˮCuSO4��ǿ���»ᷢ���ֽⷴӦ��
CuSO4 CuO + SO3��
CuO + SO3��
2SO3 2
2 SO2��+ O2��
SO2��+ O2��
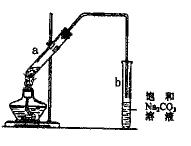
ij�о���ѧϰС���������ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ������������ֽ����ˮCuSO4��������
ʵ�鲽�裺
�ٳ�����ӦǰD �ܵ�������
�ܵ�������
�����Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ���� ��
��
�۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ��Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
��1��B���г��ֵ�������__________________________________________________��
�й����ӷ���ʽ��__________________________________________________
��2��B�ܵ������dz�ȥ��������е�SO3��ʵ������з���B�ܵ��¶��������ߣ���Ҫԭ����______________________
��3������������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m����ֽ����ˮCuSO4��������___________
ԭ����__________________________________________________________��
CuSO4
 CuO + SO3��
CuO + SO3��2SO3
 2
2 SO2��+ O2��
SO2��+ O2��ij�о���ѧϰС���������ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ������������ֽ����ˮCuSO4��������

ʵ�鲽�裺
�ٳ�����ӦǰD
 �ܵ�������
�ܵ������������Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ����
 ��
���۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ��Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
��1��B���г��ֵ�������__________________________________________________��
�й����ӷ���ʽ��__________________________________________________
��2��B�ܵ������dz�ȥ��������е�SO3��ʵ������з���B�ܵ��¶��������ߣ���Ҫԭ����______________________
��3������������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m����ֽ����ˮCuSO4��������___________
ԭ����__________________________________________________________��
��1��������ð����������ɫ�����������Է��ȣ�2�֣�
SO3 + H2O + Ba2��= BaSO4��+ 2H��
��SO3 + H2O = 2H��+SO42��, SO42�� + Ba2��= BaSO4����4�֣�
��2�� SO3����ˮ���ȣ�2�֣�
��3�����ܣ�2�֣���SO3������ȫ�ֽ�ΪSO2��O2, �Ҳ���SO2���ܽ�����Һ�У�4�֣�
SO3 + H2O + Ba2��= BaSO4��+ 2H��
��SO3 + H2O = 2H��+SO42��, SO42�� + Ba2��= BaSO4����4�֣�
��2�� SO3����ˮ���ȣ�2�֣�
��3�����ܣ�2�֣���SO3������ȫ�ֽ�ΪSO2��O2, �Ҳ���SO2���ܽ�����Һ�У�4�֣�
��

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ