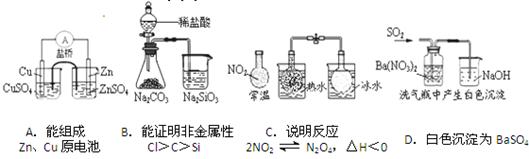
��Ŀ����
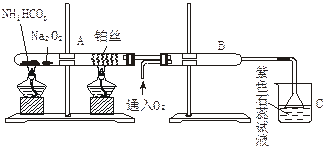
ij��ѧ����С�����������ͼ������̨������ȥ����ʾ��ʵ��װ�ã�������������ʵ�顣ͼ���ü�ͷ��ʾ��������A��ʾһ�ִ�������������壬B��ʾ��һ�����塣��Ӧ����һ��ʱ���װ�ü����к���ɫ�������ɡ�ʵ�������õ�ҩƷ�����ֻ�ܴ�����������ѡȡ��N a2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����H2O2������ˮ��
a2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����H2O2������ˮ��
����ͼ��װ�úͷ�Ӧ������ش�
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����еĸ����Ӧѡ ����ѡ��һ�ָ������������ ��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��
��4�����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ ���˷�Ӧ�ǣ����ȡ����ȣ� ��Ӧ�����ܷ�����ʲô�������֤������ж� ��
 a2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����H2O2������ˮ��
a2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����H2O2������ˮ������ͼ��װ�úͷ�Ӧ������ش�

��1�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����еĸ����Ӧѡ ����ѡ��һ�ָ������������ ��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ
 ��
����4�����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ ���˷�Ӧ�ǣ����ȡ����ȣ� ��Ӧ�����ܷ�����ʲô�������֤������ж� ��
��1��NH4HCO3 NH3����CO2����H2O����2�֣�
NH3����CO2����H2O����2�֣�
��2����ʯ�ң�1�֣�����ˮCaCl2ֻ����ˮ����������CO2����NH3Ҫ����ˮCaCl2��Ӧ�� 1�֣�
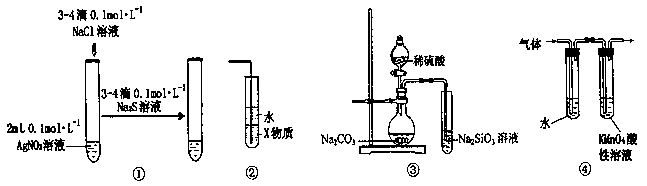
��3��2H2O2 2H2O��O2������2Na2O2��2H2O
2H2O��O2������2Na2O2��2H2O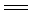 4NaOH��O2������2�֣�
4NaOH��O2������2�֣�
��4��4NH3��5O2 4NO��6H2O��2�֣������ȣ�1�֣�����Ӧ��ʼ��Ͽ����K����˿�ܼ������ֺ��ȣ�1�֣���
4NO��6H2O��2�֣������ȣ�1�֣�����Ӧ��ʼ��Ͽ����K����˿�ܼ������ֺ��ȣ�1�֣���
 NH3����CO2����H2O����2�֣�
NH3����CO2����H2O����2�֣���2����ʯ�ң�1�֣�����ˮCaCl2ֻ����ˮ����������CO2����NH3Ҫ����ˮCaCl2��Ӧ�� 1�֣�
��3��2H2O2
 2H2O��O2������2Na2O2��2H2O
2H2O��O2������2Na2O2��2H2O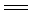 4NaOH��O2������2�֣�
4NaOH��O2������2�֣���4��4NH3��5O2
 4NO��6H2O��2�֣������ȣ�1�֣�����Ӧ��ʼ��Ͽ����K����˿�ܼ������ֺ��ȣ�1�֣���
4NO��6H2O��2�֣������ȣ�1�֣�����Ӧ��ʼ��Ͽ����K����˿�ܼ������ֺ��ȣ�1�֣�����

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ
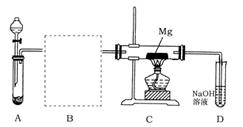
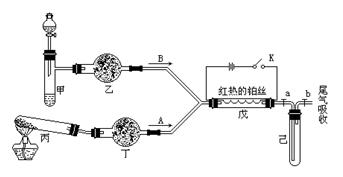
 Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺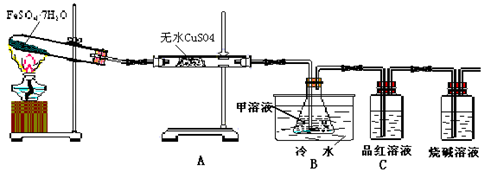
 ɫ�� ɫ��
ɫ�� ɫ�� �а��Ĵ��������ش��������⣺
�а��Ĵ��������ش��������⣺