��Ŀ����
A��B��C��һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش���
�����⣬��֪A��B��C�о�����ͬһ��Ԫ�ء�
1��DΪ�ǽ������ʣ���A��B��C֮���������ת����ϵ��
![]()
����AΪ�������ʣ� C��ˮ��Ӧ�ܹ�����D��д��C�ĵ���ʽ
����AΪ��̬�����C�ķ���ʽ������ �����������֣�
����AΪ���ĺ��������A�����к���2��̼ԭ�ӡ������£�23g A��ȫȼ�շų�683.5kJ��������д���ܱ�ʾAȼ���ȵ��Ȼ�ѧ����ʽ
����DΪ�������ʣ������Ϸ�Ӧ��Ϊ������ԭ��Ӧ����д������B�������ӵ�һ�ַ��� ��
����DΪ�BΪ��ɫ�����������Ϸ�Ӧ��Ϊ��������ԭ��Ӧ��д��B��D��Ӧ�����ӷ���ʽ ��
������������ ![]() ���������ϵ�ķ�Ӧ�����ġ�
���������ϵ�ķ�Ӧ�����ġ�
![]()
���𰸡���
�� SO2��SO3��NO2
�� C2H5OH(l) + 3O2(g) = 2CO2(g) + 3H2O(l)����H����1367 kJ/mol
�� ȡ��������Һ�������е���KSCN��Һ������Һ���˵������Һ�к���Fe3+������������Ҳ�ɸ��֡���
�� Al(OH)3 + OH�� �� AlO2�� + 2H2O

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
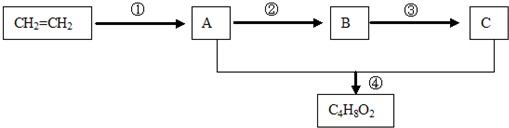
Сѧ��10����Ӧ����ϵ�д�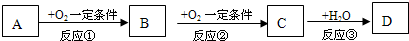
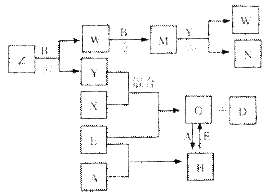 A��B��C��D��E����ѧ��ѧ���������ֵ��ʣ���֪A��B��C��D�ڳ����¾�Ϊ��̬��EΪ����������D�ֱܷ��A��B��C��һ�������»��ϣ����ɶ�Ӧ�Ļ�����X��Y��Z�����г����£�YΪҺ�壬X��ZΪ���塣�йص�ת����ϵ����ͼ��ʾ����Ӧ����������ȥ����
A��B��C��D��E����ѧ��ѧ���������ֵ��ʣ���֪A��B��C��D�ڳ����¾�Ϊ��̬��EΪ����������D�ֱܷ��A��B��C��һ�������»��ϣ����ɶ�Ӧ�Ļ�����X��Y��Z�����г����£�YΪҺ�壬X��ZΪ���塣�йص�ת����ϵ����ͼ��ʾ����Ӧ����������ȥ����