��Ŀ����
8�� A��B��C��DΪ��˳�����е�ǰ����±�ص��ʣ���ϸ�����±��е����ݣ��ش��й����⣮
A��B��C��DΪ��˳�����е�ǰ����±�ص��ʣ���ϸ�����±��е����ݣ��ش��й����⣮| ±�ص��� | �ܶȣ���״���£� | �е�/�� | �۵�/�� | �ܽ�ȣ���״���£�100gˮ�У� |
| A | 1.696 g•l-1 | -188.1 | -219.6 | -- |
| B | 3.214 g•l-1 | -34.6 | -101 | 226 cm3 |
| C | 3.119 g•cm-3 | 58.78 | -7.2 | 4.16 g |
| D | 4.93 g•cm-3 | 184.4 | 113.5 | 0.029 g |
��2��A����Է�������Ϊ38������������
��3��д��B��ˮ��Ӧ�Ļ�ѧ����ʽCl2+H2O=HCl+HClO
��4��±�ص���B��C��D���������ɴ�С��˳��ΪCl2��Br2��I2���û�ѧʽ��ʾ��
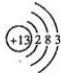
��5 ��±��Ԫ�صĵ�����H2��Ӧ�����е������仯��ͼ��ʾ������a��b��c��d�ֱ��ʾ±����ijһԪ�صĵ��ʣ�EΪ��ͬ���ʵ����ĵ�����H2��Ӧ�����е������仯����EԽ������ӦԽ������������ش��������⣺dΪI2��д��ѧʽ����ͬ�������ȵ�ͭ˿��b������ȼ�յ�����Ϊ����ȼ�գ������ػ�ɫ���̣���
���� ��1������±�ص��ʵ��۵㡢�е����ж����ʵ�״̬��
��2������M=�ѡ�Vm���Ħ������������Ħ����������Է�����������ֵ����ȣ�
��3��������Ŀ��Ϣ�ó�BΪCl2���ٸ���������ˮ��Ӧд������ʽ��
��4������ͬһ������ϵ��£����ʵ�������������
��5������±��Ԫ�صĵ������������ϵ�Խ��Խ�ѣ��Ƶ���a��b��c��d�ֱ�ΪF2��Cl2��Br2 ��I2��Ȼ���������ش�
��� �⣺��1����±�ص���A��B�ķе�ȳ��£�25�棩�ͣ������ڳ�����Ϊ��̬��±�ص���C���۵�ȳ��¸ߣ����е�ȳ��µͣ������ڳ�����ΪҺ̬��±�ص���D���۵�ȳ��¸ߣ������ڳ�����Ϊ��̬���ʴ�Ϊ��C��D��
��2����M=�ѡ�Vm=1.696g•l-1��22.4L/mol=38g/mol������A����Է�������Ϊ38���ʴ�Ϊ��38��
��3����BΪCl2����������ˮ��Ӧ��Cl2+H2O=HCl+HClO���ʴ�Ϊ��Cl2+H2O=HCl+HClO��
��4����ͬһ������ϵ��£����ʵ�����������������Cl2��Br2��I2���ʴ�Ϊ��Cl2��Br2��I2��
��5����±��Ԫ�صĵ������������ϵ�Խ��Խ�ѣ��ó�a��b��c��d�ֱ�ΪF2��Cl2��Br2 ��I2�����ȵ�ͭ˿��b������ȼ�յ�����Ϊ����ȼ�գ������ػ�ɫ���̣��ʴ�Ϊ��I2������ȼ�գ������ػ�ɫ���̣�
���� ������Ҫ������±�ص��ʵ����ʣ����ͼ��������ѧ���������⡢���������������ѶȲ���

| A�� | ������һ�ֻ���ɫ���д̼�����ζ������ | |
| B�� | ������Һ�Ⱥ���ˮ��ͬһ������ | |
| C�� | ����������ˮ | |
| D�� | ������һ�����ڸ�����ˮ�������ж����� |
| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | ||
| N2 | H2 | NH3 | ||
| �� | 1 | 3 | 0 | �ų�������23.15kJ |
| �� | 0.9 | 2.7 | 0.2 | �ų�������Q |
| A�� | �����١����з�Ӧ��ƽ�ⳣ����� | |
| B�� | ƽ��ʱ������������NH3�����������Ϊ $\frac{1}{7}$ | |
| C�� | �������д�ƽ��ʱ�ų�������Q=23.15 kJ | |
| D�� | �������������Ϊ0.5 L����ƽ��ʱ�ų�������С��23.15 kJ |
| A�� | ��ϩ�����ȩ��CH3CH2CHO����Ϊͬ���칹�� | |
| B�� | ��ϩ������ϩȩ���������ֹ����� | |
| C�� | 1 mol��ϩȩ���Ժ�2 mol���������ӳɷ�Ӧ | |
| D�� | ��ת�������б�ϩ������ԭ |
| A�� | �����ܵ�������ʶ��ǵ���� | |
| B�� | ���Dz��ܵ��������һ���Ƿǵ���� | |
| C�� | �������Һ�ܵ����ԭ������Һ�д��������ƶ������� | |
| D�� | �����ܵ����ԭ������Һ�ڵ��������µ�����������ƶ������ӵ�Ե�� |
| A�� | ���뱽ʹ֮��Ӧ | B�� | ����ϴ�����CCl4��ȡ���ú��Һ | ||
| C�� | ����KI��Һ | D�� | ����ϴ���NaOH��Һ���÷�Һ |
 ��
��