��Ŀ����
��2010?�Ĵ����������ӷ���ʽ��д��ȷ���ǣ�������
������A��������Һ�м����������������ʱ�������е�����ȫ����Ӧ��
B��������������ԣ��ܽ���������������
C������������Һ���н�ǿ�����ԣ��ܺ�ǿ�Ӧ��
D��CH2BrCOOH�е��Ȼ��������ԣ��ܺ��ռӦ����ԭ���ܱ��ǻ�ȡ����
B��������������ԣ��ܽ���������������
C������������Һ���н�ǿ�����ԣ��ܺ�ǿ�Ӧ��
D��CH2BrCOOH�е��Ȼ��������ԣ��ܺ��ռӦ����ԭ���ܱ��ǻ�ȡ����
����⣺A��������Һ�м����������������ʱ�������е������ӻ�ת��Ϊƫ������������ȫ�����ɳ�����ԭ��Ϊ��Al3++2SO42-+2Ba2++4OH-=2BaSO4��+AlO2-+2H2O����A��ȷ��
B��������������ԣ��ܽ���������������3Fe��OH��2+10H++NO3-=3Fe3++8H2O+NO������B����
C������������Һ��ǿ�Ӧ��ԭ���� NH4++H2PO4-+3OH-�T3H2O+NH3��+PO43-����C����
D��CH2BrCOOH�е��Ȼ��������ԣ��ܺ��ռӦ����ԭ���ܱ��ǻ�ȡ����CH2BrCOOH+2OH-
CH2OHCOO-+H2O+Br-����D����
��ѡA��
B��������������ԣ��ܽ���������������3Fe��OH��2+10H++NO3-=3Fe3++8H2O+NO������B����
C������������Һ��ǿ�Ӧ��ԭ���� NH4++H2PO4-+3OH-�T3H2O+NH3��+PO43-����C����
D��CH2BrCOOH�е��Ȼ��������ԣ��ܺ��ռӦ����ԭ���ܱ��ǻ�ȡ����CH2BrCOOH+2OH-
| �� |
��ѡA��
������������Ҫ����ѧ�����ӷ���ʱ����д֪ʶ�������ڿ��Ե��ȵ㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
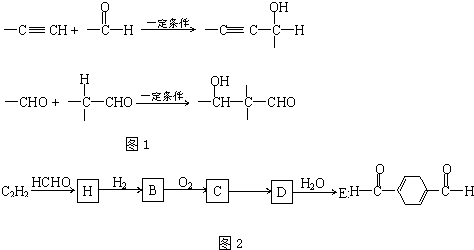


 ��
��