��Ŀ����
15��X��Y��Z��M��WΪ���ֶ�����Ԫ�أ�X��Y��Z��ԭ���������ε�����ͬ����Ԫ�أ�������������֮��Ϊ15��X��Z���γ�XZ2���ӣ�Y��M�γɵ�һ����̬��������ʹʪ��ĺ�ɫʯ����ֽ������W����������X��Y��Z��M����Ԫ��������֮�͵�1/2������˵����ȷ���ǣ�������| A�� | ԭ�Ӱ뾶��W��Z��Y��X��M | |
| B�� | Y2M4��M2Z2�ķ����к��в�ͬ��Ŀ�ĵ��� | |
| C�� | X2M2Ϊֱ���͵Ĺ��ۻ����� | |
| D�� | ��X��Y��Z��M����Ԫ���γɵĻ�����һ���������Ӽ������й��ۼ� |
���� �������������֪��X��Y��Z��M��W�����ֶ�����Ԫ�ص����У����ǰ�ԭ���������ε������еģ�����ֻ��X��Y��Z����Ԫ����ԭ���������ε�����ͬ����Ԫ�أ���X��Y��Z������������֮��Ϊ15������������ƽ��Ϊ5��X��Z���γ�XZ2��XΪ+4�ۣ�YΪ-2�ۣ����Ƴ�X��Y��Z�ֱ�ΪC��N��O����Ԫ�أ�Y��M�γɵ�һ����̬��������ʹʪ��ĺ�ɫʯ����ֽ�������������Ϊ����������MΪHԪ�أ�����W����������X��Y��Z��M����Ԫ��������֮�͵�$\frac{1}{2}$���Ƴ�W��������Ϊ$\frac{1}{2}$��6+7+8+1��=11������WΪNaԪ�أ���϶�Ӧ���ʡ�������������Լ�Ԫ��������֪ʶ�����⣮
��� �⣺�������������֪��X��Y��Z��M��W�����ֶ�����Ԫ�ص����У����ǰ�ԭ���������ε������еģ�����ֻ��X��Y��Z����Ԫ����ԭ���������ε�����ͬ����Ԫ�أ���X��Y��Z������������֮��Ϊ15������������ƽ��Ϊ5��X��Z���γ�XZ2��XΪ+4�ۣ�YΪ-2�ۣ����Ƴ�X��Y��Z�ֱ�ΪC��N��O����Ԫ�أ�Y��M�γɵ�һ����̬��������ʹʪ��ĺ�ɫʯ����ֽ�������������Ϊ����������MΪHԪ�أ�����W����������X��Y��Z��M����Ԫ��������֮�͵�$\frac{1}{2}$���Ƴ�W��������Ϊ$\frac{1}{2}$��6+7+8+1��=11������WΪNaԪ�أ�
A��ͬ������������ԭ�Ӱ뾶��С��ͬ�������ϵ���ԭ�Ӱ뾶����ԭ�Ӱ뾶Na��C��N��O��H����W��X��Y��Z��M����A����
B��N2H4��H2O2�ķ����о���18�����ӣ����Ժ�����ͬ��Ŀ�ĵ��ӣ���B����
C��X2M2ΪC2H2����Ȳ�ĽṹʽΪH-C��C-H������ֱ���͵Ĺ��ۻ������C��ȷ��
D��C��N��O��H����Ԫ���γɵĻ��������Ϊ�����ᣬ������ֻ�й��ۼ�����D����
��ѡC��
���� ���⿼����Ԫ���ƶϡ�Ԫ�������ɡ����ӽṹ��C��N��NaԪ�ػ��������ʵȣ�������ѧ���ķ��������Ŀ��飬�Ѷ��еȣ����������жϳ������Ԫ���ǽ���Ĺؼ���

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�| A�� | Ca��CaO��Ca Cl2 | B�� | O2��CuO��Cu��OH��2 | ||
| C�� | C��CO2��Na2CO3 | D�� | NaOH��Na2CO3��NaCl |
| A�� | SO42-? | B�� | Na+ | C�� | Cl- | D�� | CH3COO- |
| A�� |  | B�� | 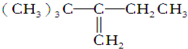 | ||
| C�� | CH2�TCHC��CH3��2CH��CH3��2 | D�� | ��CH3��3CC��CH3���TCHCH3 |
| A�� | H2O ��g���TH2��g��+$\frac{1}{2}$O2��g������H�T-485 kJ/mol | B�� | 2H2��g��+O2��g���T2H2O��g������H�T-485 kJ/mol | ||
| C�� | 2H2��g��+O2 ��g���T2H2O��g������H�T+485 kJ/mol | D�� | H2O ��g���TH2��g��+$\frac{1}{2}$O2��g������H�T+485 kJ/mol |
| A�� | Na+��K+��S2-��NO3- | B�� | Na+��NO3-��SO42-��NH3��H2O | ||
| C�� | K+��Ba2+��H2O2��Cl- | D�� | Na+��Fe2+��Cl-��SO42- |
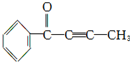 �л���M�ṹ��ͼ������M�Ľṹ�ش�M�в�����̼ԭ����9���������18��ԭ�ӹ�ƽ�森
�л���M�ṹ��ͼ������M�Ľṹ�ش�M�в�����̼ԭ����9���������18��ԭ�ӹ�ƽ�森