��Ŀ����
 ��2009?���죩��ҵ�ϵ�ⱥ��ʳ������ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�裮
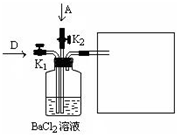
��2009?���죩��ҵ�ϵ�ⱥ��ʳ������ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�裮��1��ͼ�����ӽ���Ĥ����ⱥ��ʳ��ˮʾ��ͼ����������������������
����
����
��NaOH��Һ�ij���Ϊa
a
������ĸ�������Ʊ���ʳ��ˮ�Ľ���Ϊd
d
������ĸ������������Ӧʹ�õ�Һ����Ũ����
Ũ����
����2���ྦྷ����Ҫ����SiHCl3��ԭ�����������丱����SiCl4���ۺ������յ��㷺��ע��
��SiCl4���������̿�ڣ�����ά��Ҫԭ����ͬ��������Ϊ������SiCl4��H2��O2��Ӧ�����������֣���ѧ����ʽΪ
SiCl4+2H2+O2
SiO2+4HCl
| ||
SiCl4+2H2+O2
SiO2+4HCl
��
| ||
��SiCl4��ת��ΪSiHCl3��ѭ��ʹ�ã�һ�������£���20L�����ܱ������еķ�Ӧ��3SiCl4��g��+2H2��g��+Si��g��
 4SiHCl3��g����ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ
4SiHCl3��g����ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ0.351
0.351
kg����3��������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0kg������������
134.4
134.4
M3����״��������������1����ⱥ��ʳ��ʱ����������Cl-��OH-�ŵ磬Cl-�ķŵ�����ǿ��OH-�����������ķ���ʽΪ��2Cl--2e-�TCl2����������2H++2e-�TH2����H2��2NaOH��������NaOH��Һ�ij���Ϊa��Cl2�����������Ʊ���ʳ��ˮ���������룬Ҫ����Cl2��Ҫ�����Ը���������Ը������
��2����SiCl4��H2��O2��Ӧ�����������֣����ά����Ҫ�ɷ���SiO2��H��ClԪ�ر�����һ�����У�H��ClԪ�ؽ�ϳ�HCl��Ȼ����ƽ���ɣ�
���������η�����������ƽ��ʱH2��SiHCl3���ʵ���Ũ�ȣ��������ʼ���ʵ������ٸ���2NaCl+2H2O
Cl2��+H2��+2NaOH��������������Ĵ�NaCl��������
��3�����ݵ�ʧ�����غ㣬NaClת��ΪNaClO3��ʧȥ�ĵ��ӵ���H2Oת��ΪH2���õ��ĵ��ӣ��������Ƶ�������������Ƶ����ʵ������������NaClת��ΪNaClO3��ʧȥ�ĵ��ӵ����ʵ��������������������ڱ�״���µ������
��2����SiCl4��H2��O2��Ӧ�����������֣����ά����Ҫ�ɷ���SiO2��H��ClԪ�ر�����һ�����У�H��ClԪ�ؽ�ϳ�HCl��Ȼ����ƽ���ɣ�
���������η�����������ƽ��ʱH2��SiHCl3���ʵ���Ũ�ȣ��������ʼ���ʵ������ٸ���2NaCl+2H2O
| ||
��3�����ݵ�ʧ�����غ㣬NaClת��ΪNaClO3��ʧȥ�ĵ��ӵ���H2Oת��ΪH2���õ��ĵ��ӣ��������Ƶ�������������Ƶ����ʵ������������NaClת��ΪNaClO3��ʧȥ�ĵ��ӵ����ʵ��������������������ڱ�״���µ������
����⣺��1����ⱥ��ʳ��ʱ����������Cl-��OH-�ŵ磬Cl-�ķŵ�����ǿ��OH-��
������2Cl--2e-�TCl2����
������2H++2e-�TH2����
�ܷ�ӦΪ��2NaCl+2H2O
Cl2��+H2��+2NaOH��
�����������ӷŵ磬������������ʹ����������Ũ�����������Ӻ����������ӵ��������������ң�����a���ڵ�����Һ��������������Һ��
�����������ӷŵ磬������������ʹ������Ũ�����ߣ�ͨ�������ӽ���Ĥ���������ң�����d���Ӧ���뾫�Ʊ���ʳ��ˮ��
Ҫ����Cl2��Ҫ�����Ը����Ũ�����P2O5�ȣ����Ը������ˮCaCl2��
��2����SiCl4��H2��O2��Ӧ�����������֣����ά����Ҫ�ɷ���SiO2��H��ClԪ�ر�����һ�����У�H��ClԪ�ؽ�ϳ�HCl��Ȼ����ƽ���ɣ�
�����Ļ�ѧ����ʽΪ��SiCl4+2H2+O2
SiO2+4HCl��
����3SiCl4��g��+2H2��g��+Si��s�� 4SiHCl3��g��
4SiHCl3��g��
��ʼ����mol�� n 0
�仯����mol�� 2x x 4x
ƽ������mol�� n-2x 4x
4x=0.020mol/L��20L=0.4mol��x=0.1mol��
n-2x=0.140mol/L��20L=2.8mol��n=3.0mol��
��2NaCl+2H2O
Cl2��+H2��+2NaOH��
2mol 1mol
=
�� m��NaCl��=351g=0.351kg��
��3����NaClת��ΪNaClO3��ʧȥ������Ϊ6��H2Oת��ΪH2���õ��ĵ�����Ϊ2��
�������H2���ΪVm3��
�ɵ�ʧ�����غ�ã�6��
=2��
��
V=134.4m3��
�ʴ�Ϊ����1����������a��d��Ũ���
��2����SiCl4+2H2+O2
SiO2+4HCl����0.351��
��3��134.4��
������2Cl--2e-�TCl2����
������2H++2e-�TH2����
�ܷ�ӦΪ��2NaCl+2H2O
| ||
�����������ӷŵ磬������������ʹ����������Ũ�����������Ӻ����������ӵ��������������ң�����a���ڵ�����Һ��������������Һ��
�����������ӷŵ磬������������ʹ������Ũ�����ߣ�ͨ�������ӽ���Ĥ���������ң�����d���Ӧ���뾫�Ʊ���ʳ��ˮ��
Ҫ����Cl2��Ҫ�����Ը����Ũ�����P2O5�ȣ����Ը������ˮCaCl2��
��2����SiCl4��H2��O2��Ӧ�����������֣����ά����Ҫ�ɷ���SiO2��H��ClԪ�ر�����һ�����У�H��ClԪ�ؽ�ϳ�HCl��Ȼ����ƽ���ɣ�
�����Ļ�ѧ����ʽΪ��SiCl4+2H2+O2
| ||
����3SiCl4��g��+2H2��g��+Si��s��
 4SiHCl3��g��
4SiHCl3��g����ʼ����mol�� n 0
�仯����mol�� 2x x 4x
ƽ������mol�� n-2x 4x
4x=0.020mol/L��20L=0.4mol��x=0.1mol��
n-2x=0.140mol/L��20L=2.8mol��n=3.0mol��
��2NaCl+2H2O
| ||
2mol 1mol
| 2��58.5g |
| 1mol |
| m(NaCl) |
| 3mol |
��3����NaClת��ΪNaClO3��ʧȥ������Ϊ6��H2Oת��ΪH2���õ��ĵ�����Ϊ2��
�������H2���ΪVm3��
�ɵ�ʧ�����غ�ã�6��
| 213��103g |
| 116.5gmol-1 |
| Vm3��103L?m-3 |
| 22.4L?mol-1 |
V=134.4m3��
�ʴ�Ϊ����1����������a��d��Ũ���
��2����SiCl4+2H2+O2
| ||
��3��134.4��
���������⿼���⡢�ȼҵ����ѧƽ��ļ��㡢��ѧ����ʽ��д����ѧ�����֪ʶ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
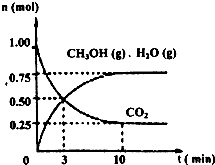 2009��12��7��һ18���ڵ������籾�����ٿ������Ϲ�������飬��δ��Ӧ������仯��ȫ���ж�ǩ���µ�Э�飮����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���
2009��12��7��һ18���ڵ������籾�����ٿ������Ϲ�������飬��δ��Ӧ������仯��ȫ���ж�ǩ���µ�Э�飮����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���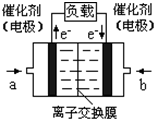 ��ͼ����a��ͨ�����
��ͼ����a��ͨ�����