��Ŀ����
Ϊ�˲ⶨ�������ƹ�������m g��̼���Ƶ������������ס�����λͬѧ�ֱ���������µ�ʵ�鷽����
(��)��ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ�����ˡ�ϴ�ӡ���ɣ������ù���n g��
(1)�������̼���Ƶ���������Ϊ(��m��n��ʾ)________��
(2)��ͬѧϴ�ӳ����IJ�����________________��
(3)Ca2+��Ba2+������ʹCO32��������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ���ǣ�
��________________________��
��________________________��
(��)��ͬѧ�ķ�����ͼ��ʾ��
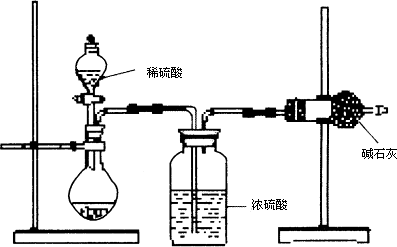
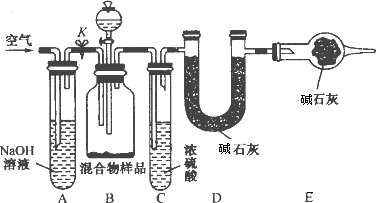
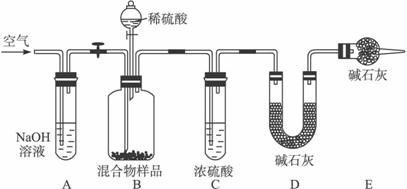
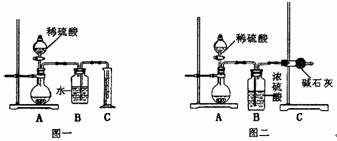
(1)������ͬѧ��ʵ��װ��ͼ��������ÿ��ʵ���У���ɲⶨ��������Ҫ����________�γ���������
(2)���ظ���ȷ���������Σ�������ݳ����˽ϴ��ƫ�����Ϊ��Ҫԭ�������(��д����)����________________________________��
��_______________________________________��
��_______________________________________��
������
����(��)(1)10600 n/197 m��
����(2)�ز�������������еij����ϼ�����ˮ����û����������ʹ��ȫ���˳����ظ�2��3��
����(3)�ټ���BaCl2��Һ�����ɵij�������������������С��
�����ڹ�����Ca2+����OH�������ܵ�Ca(OH)2����Ӱ��ⶨ���
����(��)(1)4
����(2)��װ����ԭ�п����е�CO2û���ų�������Ҳ����ʯ������
�����ڷ�Ӧ��ɺ�װ���е�CO2û��ȫ������ʯ������
�����ۿ����е�CO2��ˮ��������ʯ������

 ��У����ϵ�д�
��У����ϵ�д�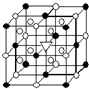 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������