��Ŀ����
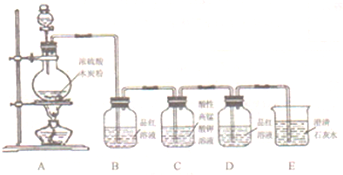
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ʵ�飬ʵ��װ��������ʾ��ʵ�鲽�輰�������£�����������ͼװ�ã���������ԣ��ټ����Լ����ڼ����Թ�A��B��Ʒ����Һ��ɫ��Ϩ��ƾ��ƣ��۽�Cu˿���ϳ鶯ʹ�¶��뿪Һ�森
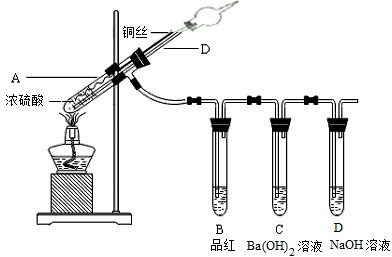
��1�����裨2�����Թ�A�����ʷ�����Ӧ�Ļ�ѧ����ʽ______��
��2����ʢ��BaCl2��Һ���Թ�C�У����˵��ܿ��������⣬���������������Թ��е���Һȡ�����μ���ˮ��������������______��������Ӧ�����ӷ���ʽΪ______��______��
��3���Թ�D��NaOH��Һ��������______��
��4���Թ�B��Ʒ����Һ��ɫ˵��SO2����Ư���ԣ���֪Cl2Ҳ��ʹƷ����ɫ��������ƿ�ֱ�SO2��Cl2Ư����Ʒ����Һ���������һ����ʵ�������м���д�����������������ۣ�______��

��1�����裨2�����Թ�A�����ʷ�����Ӧ�Ļ�ѧ����ʽ______��
��2����ʢ��BaCl2��Һ���Թ�C�У����˵��ܿ��������⣬���������������Թ��е���Һȡ�����μ���ˮ��������������______��������Ӧ�����ӷ���ʽΪ______��______��
��3���Թ�D��NaOH��Һ��������______��
��4���Թ�B��Ʒ����Һ��ɫ˵��SO2����Ư���ԣ���֪Cl2Ҳ��ʹƷ����ɫ��������ƿ�ֱ�SO2��Cl2Ư����Ʒ����Һ���������һ����ʵ�������м���д�����������������ۣ�______��
��1��ͭ��Ũ���Ṳ����������ͭ�����������ˮ����Ӧ����ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
��2�������ܹ������������������ԭ��Ӧ������������ӣ�����������뱵���ӷ�Ӧ�������ᱵ��������Ӧ�����ӷ���ʽΪ��SO2+H2O+Cl2�T2Cl-+4H++SO42-��SO42-+Ba2+=BaSO4�����������Թ��еμ���ˮ����Һ�����ǣ�
�ʴ�Ϊ����Һ����ǣ�SO2+H2O+Cl2�T2Cl-+4H++SO42-��SO42-+Ba2+=BaSO4����
��3�����������ж���Ϊ�˷�ֹ�����Ⱦ����Ҫʹ��β������װ�ã�����������������Һ���ն���Ķ�������
�ʴ�Ϊ������SO2��ֹ��Ⱦ��
��4������������Ư��Ϊ��ʱ�ԣ�������ɵ���ɫ���ʲ��ȶ�����ʵ�鷽��Ϊ����ɫ�����Һ���ȣ������»ָ���ɫ����ͨ���������SO2������Ϊ��ɫ����ͨ���������Cl2��
�ʴ�Ϊ������ɫ�����Һ���ȣ������»ָ���ɫ����ͨ���������SO2������Ϊ��ɫ����ͨ���������Cl2��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��2�������ܹ������������������ԭ��Ӧ������������ӣ�����������뱵���ӷ�Ӧ�������ᱵ��������Ӧ�����ӷ���ʽΪ��SO2+H2O+Cl2�T2Cl-+4H++SO42-��SO42-+Ba2+=BaSO4�����������Թ��еμ���ˮ����Һ�����ǣ�
�ʴ�Ϊ����Һ����ǣ�SO2+H2O+Cl2�T2Cl-+4H++SO42-��SO42-+Ba2+=BaSO4����
��3�����������ж���Ϊ�˷�ֹ�����Ⱦ����Ҫʹ��β������װ�ã�����������������Һ���ն���Ķ�������
�ʴ�Ϊ������SO2��ֹ��Ⱦ��
��4������������Ư��Ϊ��ʱ�ԣ�������ɵ���ɫ���ʲ��ȶ�����ʵ�鷽��Ϊ����ɫ�����Һ���ȣ������»ָ���ɫ����ͨ���������SO2������Ϊ��ɫ����ͨ���������Cl2��
�ʴ�Ϊ������ɫ�����Һ���ȣ������»ָ���ɫ����ͨ���������SO2������Ϊ��ɫ����ͨ���������Cl2��

��ϰ��ϵ�д�
�����Ŀ