��Ŀ����
(9��) (1)���з�Ӧ����Ƴɻ�ѧ��ת��Ϊ���ܵ�װ������ ��
A Zn��CuSO4��ZnSO4��Cu��������B NaOH+HCl=NaCl+H2O
(2)����(1)�еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ��������������͵������Һ����д���缫��Ӧʽ��
������Ӧ�� ������Ӧ�� .

(3)ij������������������ͬ����������������36���������ķ���ʽΪ____________����д������ͬ���칹������4�ֲ�ͬ�е��һ�ȴ���ĸ����Ľṹ��ʽ����������
��
A Zn��CuSO4��ZnSO4��Cu��������B NaOH+HCl=NaCl+H2O
(2)����(1)�еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ��������������͵������Һ����д���缫��Ӧʽ��
������Ӧ�� ������Ӧ�� .

(3)ij������������������ͬ����������������36���������ķ���ʽΪ____________����д������ͬ���칹������4�ֲ�ͬ�е��һ�ȴ���ĸ����Ľṹ��ʽ����������
��
(1)���� A ��1�֣�
(2) Cu2++2e-=Cu ��1�֣�Zn-2e-=Zn2+����1�֣�����������ֻҪ�������ɣ���2�֣�
(3)C5H12 ��1�֣� ��1�֣� 2-�����飨�������飩��1�֣�
(2) Cu2++2e-=Cu ��1�֣�Zn-2e-=Zn2+����1�֣�����������ֻҪ�������ɣ���2�֣�
(3)C5H12 ��1�֣� ��1�֣� 2-�����飨�������飩��1�֣�
��1����Ϊ���ӵĶ����ƶ������γɵ���������ֻ��������ԭ��Ӧ������Ƴ�ԭ��أ���ѡ��A��ȷ��
��2�����ݷ�Ӧʽ��֪��п�ǻ�ԭ����ʧȥ���ӣ���������ͭ���ӵõ����ӣ��������õ������������缫��Ӧʽ�ֱ�Ϊ������Ӧ�� Zn-2e-=Zn2+��������Ӧ��Cu2++2e-=Cu��װ��ͼ��ͼ��ʾ��
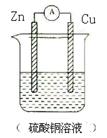
��3��������ܶ�֮������Ӧ����Է�������֮�ȣ�������������Է���������36��2��72�����Ը���������ͨʽCnH2n��2��֪������ʽΪC5H12��������3��ͬ���칹�壬������������3����ԭ�ӡ���������4����ԭ�ӣ���������1�֡�
��2�����ݷ�Ӧʽ��֪��п�ǻ�ԭ����ʧȥ���ӣ���������ͭ���ӵõ����ӣ��������õ������������缫��Ӧʽ�ֱ�Ϊ������Ӧ�� Zn-2e-=Zn2+��������Ӧ��Cu2++2e-=Cu��װ��ͼ��ͼ��ʾ��

��3��������ܶ�֮������Ӧ����Է�������֮�ȣ�������������Է���������36��2��72�����Ը���������ͨʽCnH2n��2��֪������ʽΪC5H12��������3��ͬ���칹�壬������������3����ԭ�ӡ���������4����ԭ�ӣ���������1�֡�

��ϰ��ϵ�д�
�����Ŀ


 Cu2+
Cu2+

