��Ŀ����
����Ŀ��ʵ��������500mL 0.2mol/L��NaOH��Һ��
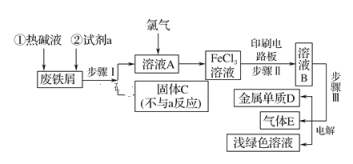
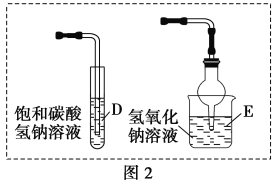
(1)����ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_____________(�����)����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��____________��
(2)��д���������еĿհף�
���岽�����£�
�ټ�����Ҫ����NaOH���������___________g��
����������ƽ����NaOH���壻
�۽��ƺõ�NaOH��������ձ��У�����������ˮ�ܽ⡢���裬��_________�����£�
�ܽ�NaOH��Һ�ز�����ע��____________�У�
������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲ��ע������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�
������ˮע������ƿ��Һ����̶�����_______cmʱ������____________�μ�����ˮ��Һ���ڿ̶������У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ�
(3)����ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L��ԭ�������_____________
A.ʹ����ֽ����NaOH���壻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ�
C.����ƿ��ԭ������������ˮ��
D.����ʱ���õ��������⣻
E.δ��ȴֱ��ת��������ƿ��������á�
���𰸡�C �ձ� ������ 4.0 ��ȴ 500mL����ƿ 1��2 ��ͷ�ι� AB
��������
(1)����ʵ������IJ��裨���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������Լ�ÿ��������Ҫ����ȷ����Ӧ����������
(2)����m=c��V��M������Ҫ���ʵ���������������һ�����ʵ���Ũ����Һ��һ�㲽��ȷ��ʹ�õ�������������Ҫ��
(3)�������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(1)����һ�����һ�����ʵ���Ũ�ȵ���ҺҪ��һ�����������ƿ�н��У�������ƽ�������ʣ�����Ͳ��ȡˮ�����ձ��н����ܽ⣬Ϊ�ٽ������ܽ⣬Ҫ�ò��������裬����Һ�ָ������º�ͨ������������ת����������ƿ�У�����ý�ͷ�ιܶ��ݡ�������ͼ��ʾ�����У�����������Һ�϶�����Ҫ���Ƿ�Һ©���������C������Ҫʹ�õIJ����������ձ�����������
(2)������500mL 0.2mol/LNaOH��Һ��Ҫ�������Ƶ�����Ϊ��m(NaOH)= 0.2mol/L��0.5L ��40g/mol=4.0g��
����������ƽ����NaOH���壻
����������ƿҪ�����Ƶ���Һ���¶������£�����Ҫ���ƺõ�NaOH��������ձ��У�����������ˮ�ܽ⡢���裬����ȴ�����£�
�ܽ�NaOH��Һ�ز�����ע��500mL����ƿ�У�
������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲ��ע������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�
������ˮע������ƿ��Һ����̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ���ڿ̶������У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ��͵õ�500mL 0.2mol/LNaOH��Һ��
(3)����ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L��С��0.2mol/L��
A.ʹ����ֽ����NaOH���壬������������NaOH����մ����ֽ�ϣ��������ʵ�����ƫ�٣���ȡ�����������������ʵ���Ҳ��ƫС��ʹ������Һ��Ũ��ƫ�ͣ�A�������⣻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ��������ʵ��������٣�ʹ������Һ��Ũ��ƫ�ͣ�B�������⣻
C.����ƿ��ԭ������������ˮ����Ӱ�����ʵ���������Һ���������˶����Ƶ���ҺŨ����Ӱ�죬C���������⣻
D.����ʱ���õ��������⣬ʹ���ʵ�����ƫ����������Ƶ���ҺŨ��ƫ�ߣ�D���������⣻
E.δ��ȴֱ��ת��������ƿ��������ã�����Һ�ָ�������ʱ����Һ�����ƫС���������Ƶ���ҺŨ��ƫ�ߣ�E���������⣻
�ʺ���ѡ����AB��

����Ŀ��(1)��֪Ksp[Cu(OH)2]��2.2��10��20��Ksp[Fe(OH)3]��2.6��10��39�������£�ij����CuCl2��Һ�к���������FeCl3��Ϊ�˵õ�������CuCl2��2H2O���壬Ӧ����___________(��������Ļ�ѧʽ)��������Һ��pH��4��ʹ��Һ�е�Fe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��________�����˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl2��2H2O���塣
(2)ij̼�ظֹ�¯��ˮ������Ҫ�ɷ���̼��ơ�����ơ�������þ�����⡢��������ȡ�ˮ���輰ʱ��ϴ��ȥ����ϴ�������£�
��.����NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
��.�ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
��.��ϴ��Һ�м���Na2SO3��Һ��
��.��ϴ��꣬��NaNO2��Һ�ۻ���¯��
����ϡ�����ܽ�̼��Ƶ����ӷ���ʽ��_____________________________��
����֪��25 ��ʱ�й����ʵ��ܶȻ�
���� | CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
Ksp | 2.8��10��9 | 9.1��10��6 | 1.8��10��11 | 6.8��10��6 |
�������ݣ���ϻ�ѧƽ��ԭ��������ϴCaSO4�Ĺ���________________�������ܽ�ƽ�����ʽ�ͱ�Ҫ��������������˵�������ڲ������ݹ����л��ᷢ����ӦMgCO3(s)��2OH��(aq)![]() Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
�۲�����У�����Na2SO3��Һ��Ŀ����_______________________________��