��Ŀ����
����Ŀ����ҵ��������һ����Ҫ��Ӧ�ǣ�CO(g)+H2O(g)=CO2(g)+H2(g)��
��֪��25��ʱ����C(s)+![]() O2(g)
O2(g)![]() CO(g) H4=-111kJ/mol
CO(g) H4=-111kJ/mol
��H2(g)+![]() O2(g)=H2(g) H2=-242kJ/mol
O2(g)=H2(g) H2=-242kJ/mol
��C(s)+O2(g)=CO2(g) H2=-394kJ/mol
����˵������ȷ���ǣ� ��
A.25��ʱ��![]()
B.����ѹǿ����Ӧ�ٵ�ƽ�����淴Ӧ�����ƣ�ƽ�ⳣ��K��С
C.��Ӧ�ٴﵽƽ��ʱ��ÿ����![]() ��ͬʱ����0.5molO2
��ͬʱ����0.5molO2
D.��Ӧ�ڶϿ�2molH2��1molO2�еĻ�ѧ�������յ��������γ�4molO-H�����ų���������484kJ
���𰸡�B
��������
A.��25��ʱ����C(s)+![]() O2(g)
O2(g)![]() CO(g) H4=-111kJ/mol����H2(g)+
CO(g) H4=-111kJ/mol����H2(g)+![]() O2(g)=H2(g) H2=-242kJ/mol��C(s)+O2(g)=CO2(g) H2=-394kJ/mol����ϸ�˹���ɿ�֪��-��-���õ�CO(g)+H2O(g)=CO2(g)+H2(g)��H=-41kJ/mol����A��ȷ��
O2(g)=H2(g) H2=-242kJ/mol��C(s)+O2(g)=CO2(g) H2=-394kJ/mol����ϸ�˹���ɿ�֪��-��-���õ�CO(g)+H2O(g)=CO2(g)+H2(g)��H=-41kJ/mol����A��ȷ��
B.����ѹǿ����Ӧ����ƽ�����淴Ӧ�����ƶ����¶Ȳ��䣬��ƽ�ⳣ��K���䣬��B����
C.ƽ��ʱ��ͬ���ʵ����ʵ����ı仯��֮�ȵ��ڻ�ѧ������֮�ȣ������ﵽƽ��ʱ��ÿ����1molCO��ͬʱ����0.5molO2����C��ȷ��
D.��Ӧ��Ϊ���ȷ�Ӧ���ʱ���ڶ��ѻ�ѧ�����յ�������ȥ�ɼ��ͷŵ������������ʵ��������������ȣ���Ӧ���Ͽ�2molH-H��1molO=O�еĻ�ѧ�������յ��������γ�4molO-H�����ų���������484kJ����D��ȷ��
��ѡB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������Ƶ�ʵ�鷽���ܴﵽ��Ӧʵ��Ŀ������������
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | �����ʷ����˱��� | ������Һ�м���CuCl2��(NH4)2SO4������Һ |
B | ֤����Ӧ���ʻ��淴Ӧ��Ũ�ȵ�������ӿ� | ��3mLϡ������������п��Ӧ�������������ʽ�����Ȼ�����1mL 1mol��L��1CuSO4��Һ��Ѹ�ٲ����϶����� |
C | �Ƚ�Ksp(BaCO3)��Ksp(BaSO4) | �����£���Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�����ݲ��� |
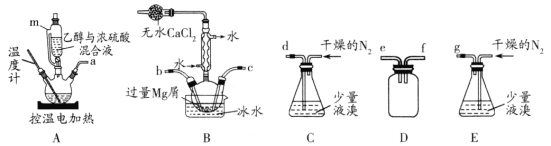
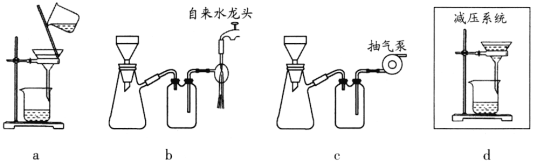
D | ͨ���۲�Һ����жϸ�װ�õ������� |
|
A. A B. B C. C D. D