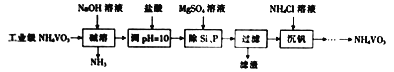
��Ŀ����
����Ŀ�������£����ȡ0.1mol/LHA��Һ��0.1mol/LNaOH��Һ�������ϣ����Ի�Ϻ���Һ����ı仯������û����Һ��pH=8���Իش��������⣺
��1�������ҺpH=8��ԭ���� �������ӷ���ʽ��ʾ��
��2�������Һ����ˮ�������c(OH-) (������������������������С����)0.1mol/LNaOH��Һ����ˮ�������c(OH-)
��3����������Һ��������ʽ�ľ�ȷ����������������֣���
c(Na+)- c(A-)= mol/L, c(OH-)- c(HA)= mol/L
(4)����������PH=2����HA��Һ��PH��12��NaOH��Һ�������Ϻ���������ҺPH 7 (������������������������С����)
��5����֪NH4A��ҺΪ��������֪HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��PH 7(������������������������С����)����ͬ�¶��µ�Ũ�ȵ�������������Һ��PH�ɴ�С��˳�������� ���������
A.NH4HCO3 B.NH4A C.(NH4)2SO4 D.NH4Cl
���𰸡���1��A����H2O![]() HA��OH�� ��2������
HA��OH�� ��2������
��3��9.9��10��7��1��10��8��4��С�ڣ�5��������ABDC
��������
�����������1����Ӧ������ǿ�������Σ��������ˮ����Һ�Լ�������A����H2O![]() HA��OH����
HA��OH����
��2�������Һ��ˮ�ĵ����ܵ��ٽ���ͬŨ��NaOH��Һ��ˮ�ĵ����ܵ����ƣ�
��3�������Һ��pH��8��c(H+)��10-8 mol��L��1��c(OH-)��Kw/ c(H+)��10-6mol��L��1�����ݵ���غ�c(Na��) +c(H+)��c(A��)+c(OH-)������c(Na��)-c(A��)��c(OH-)-c(H+)=9.9��10��7 mol/L�����������غ��֪ c(OH-)-c(HA)��c(H+)��10-8mol/L
��4��HAΪ����������������pH��2����HA��Һ��pH��12��NaOH��Һ�������Ϻ�HA������������ҺpHС��7��
��5����֪NH4A��Һ���ԣ�˵��NH3H2O��HA�ĵ���̶���ͬ����֪HA��Һ�ӵ�Na2CO3��Һ��������ų���˵��HA������ǿ��̼�ᣬHA�ĵ���̶ȴ���̼�ᣬ����˭ǿ��˭�ԣ���(NH4)2CO3��Һ��pH����7����ͬ�¶�����ͬŨ�ȵ���������Һ�У�笠�����Ũ������������泥�̼��������Ӻ�笠�������ٽ�ˮ�⣬����笠�����Ũ����С��笠����Ӻ�A������ٽ�ˮ�⣬����ٽ��̶�С��̼����泥��Ȼ����笠�������ˮ�⣬��ˮ��̶�С��NH4A�������⼸����Һ��c��NH4+���ɴ�С��˳������ΪABDC��

����Ŀ�����и������ʷ�����ȷ���ǣ� ��
����� | �ǵ���� | ������ | |
A | H2SO4 | Cl2 | NaCl |
B | NaOH | �ƾ� | N2 |
C | CuSO4 | ˮ�� | CO2 |
D | BaSO4 | ���� | KClO3 |
A.A
B.B
C.C
D.D