��Ŀ����
ij�ᾧˮ���ﺬ�����������Ӻ�һ�������ӣ���ȡ����������Ϊ45.3 g�ĸýᾧˮ����ֱ��Ƴ���Һ��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����������ݳ����������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ���������Ⱥƿ��ռ���2.24 L������(��״��)������ɫ�������ٲ�������ʧ����һ����μ���Ba(OH)2��Һ����ʼ�������ƣ����������а�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6 g.
��ش��������⣺
(1)�ýᾧˮ�����к��е�������������________��________����������________��
(2)�ýᾧˮ����Ļ�__________________________________________________��
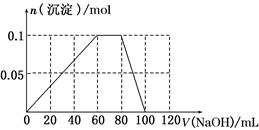
(3)��������������Һ�м����NaOH��Һ�����ʵ���Ũ��Ϊ5 mol��L��1��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��
��ش��������⣺
(1)�ýᾧˮ�����к��е�������������________��________����������________��
(2)�ýᾧˮ����Ļ�__________________________________________________��
(3)��������������Һ�м����NaOH��Һ�����ʵ���Ũ��Ϊ5 mol��L��1��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��

��(1) NH4������Al3������SO42��
(2)NH4Al(SO4)2��12H2O[��(NH4)2SO4��Al2(SO4)3��24H2O]
(3)
(2)NH4Al(SO4)2��12H2O[��(NH4)2SO4��Al2(SO4)3��24H2O]
(3)

(1)��ʹʪ���ɫʯ����ֽ������������NH3����ˮ��������NH4��������NaOH��Һ���ȳ��ֳ������������ʧ����ˮ��������Al3��.����Ba(OH)2�ȳ��ֳ�����������ܽ⣬�ܽⲻ�꣮û���ܽ�ij���������HNO3˵����SO42����
(2)n(SO42��)��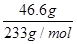 ��0.2 mol
��0.2 mol
n(NH4��)�� ��0.1 mol
��0.1 mol
�������ӻ����������������ӵ��ƽ���ԭ����n(NH4��)��3n(Al3��)��2n(SO42��)�ɵã�
n(Al3��)��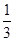 (2��0.2 mol��0.1 mol)��0.1 mol
(2��0.2 mol��0.1 mol)��0.1 mol
n(H2O)��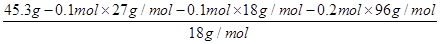 ��1.2 mol
��1.2 mol
�ýᾧˮ����Ļ�ѧʽΪNH4 Al(SO4)2��12H2O[��(NH4)2SO4��Al2(SO4)3��24H2O]
(3)Al3����ȫ��������V(NaOH)�� ��0.060 L��60 mL
��0.060 L��60 mL
NH4��ת��ΪNH3��H2O����V(NaOH)��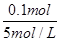 ��0.020 L��20 mL
��0.020 L��20 mL
Al(OH)3��ȫ�ܽ�����V(NaOH)��20 moL��
(2)n(SO42��)��
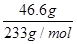 ��0.2 mol
��0.2 moln(NH4��)��
 ��0.1 mol
��0.1 mol�������ӻ����������������ӵ��ƽ���ԭ����n(NH4��)��3n(Al3��)��2n(SO42��)�ɵã�
n(Al3��)��
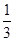 (2��0.2 mol��0.1 mol)��0.1 mol
(2��0.2 mol��0.1 mol)��0.1 moln(H2O)��
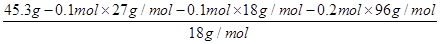 ��1.2 mol
��1.2 mol�ýᾧˮ����Ļ�ѧʽΪNH4 Al(SO4)2��12H2O[��(NH4)2SO4��Al2(SO4)3��24H2O]
(3)Al3����ȫ��������V(NaOH)��
 ��0.060 L��60 mL
��0.060 L��60 mLNH4��ת��ΪNH3��H2O����V(NaOH)��
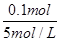 ��0.020 L��20 mL
��0.020 L��20 mLAl(OH)3��ȫ�ܽ�����V(NaOH)��20 moL��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��Һ�������е�
��Һ�������е�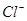 ���Ա�20 mL 0��5
���Ա�20 mL 0��5 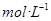 ��
��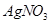 ��Һȫ����������
��Һȫ����������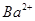 �����ʵ���Ũ��Ϊ
�����ʵ���Ũ��Ϊ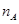 Ϊ�����ӵ���������ֵ������˵����ȷ����
Ϊ�����ӵ���������ֵ������˵����ȷ����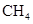 �к���4
���4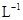
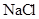 ��Һ����
��Һ����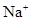
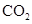 ���
���