��Ŀ����
����A��E����ѧ��ѧʵ���г����ļ���ʵ��װ�ã��Իش��������⣺
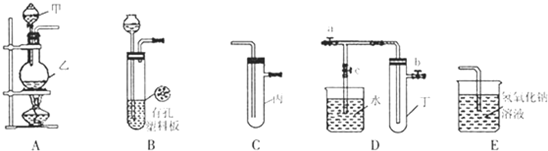
��1����Ϊ��������ʵ����ѡ���ʵ�װ�ã��������ĸ��
������װ����
������װ����
����ʯ�����Ȼ�粒�����NH3ѡ��
��2�����г��������ʵķ�����ᴿ��װ����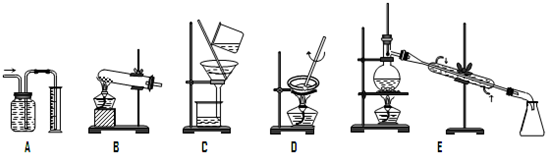
��1����Ϊ��������ʵ����ѡ���ʵ�װ�ã��������ĸ��
������װ����
A
A
�ڹ���װ����C
C
������װ����
D
D
������װ����E
E
����ʯ�����Ȼ�粒�����NH3ѡ��
B
B
��I2��CCl4��Һ����ȡI2ѡ��E
E
��2�����г��������ʵķ�����ᴿ��װ����
CDE
CDE
������ţ�������д��һ��Ҫ�õ���Һ©�����з�����ᴿ�Ļ���ʵ�������������ȡ����Һ
��ȡ����Һ
��
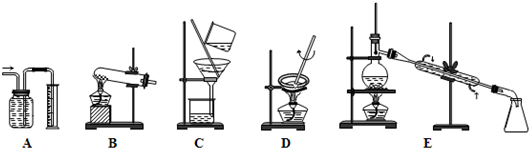
��������1������Aװ��������װ�ã�
����Bװ���ǹ���+����
�����װ�ã�
����Cװ���ǹ���װ�ã�
����Dװ��������װ�ã�
����Eװ��������װ�ã�
��2�����ݳ��������ʵķ�����ᴿ�ķ��������ˡ�����������Һ����ȡ�ȣ�������ȡ�ͷ�Һ����Ҫ��Һ©����
����Bװ���ǹ���+����
| ���� |
����Cװ���ǹ���װ�ã�
����Dװ��������װ�ã�
����Eװ��������װ�ã�
��2�����ݳ��������ʵķ�����ᴿ�ķ��������ˡ�����������Һ����ȡ�ȣ�������ȡ�ͷ�Һ����Ҫ��Һ©����
����⣺��1������Aװ��������װ�ã��ʴ�Ϊ��A��
����Cװ���ǹ���װ�ã��ʴ�Ϊ��C��
����Dװ��������װ�ã��ʴ�Ϊ��D��
����Eװ��������װ�ã��ʴ�Ϊ��E��
������ʯ�����Ȼ�粒�����NH3�ǹ���+����
���壬�ʴ�Ϊ��B��
����CCl4�ӷ�����������ķ��������CCl4���ʴ�Ϊ��E��
��2�����������ʵķ�����ᴿ�ķ��������ˡ��������ᾧ������Һ����ȡ�ȣ��ʴ�Ϊ��CDE��
����ȡ�ͷ�Һ����Ҫ��Һ©�����ʴ�Ϊ����ȡ���Һ��
����Cװ���ǹ���װ�ã��ʴ�Ϊ��C��
����Dװ��������װ�ã��ʴ�Ϊ��D��
����Eװ��������װ�ã��ʴ�Ϊ��E��
������ʯ�����Ȼ�粒�����NH3�ǹ���+����
| ���� |
����CCl4�ӷ�����������ķ��������CCl4���ʴ�Ϊ��E��
��2�����������ʵķ�����ᴿ�ķ��������ˡ��������ᾧ������Һ����ȡ�ȣ��ʴ�Ϊ��CDE��
����ȡ�ͷ�Һ����Ҫ��Һ©�����ʴ�Ϊ����ȡ���Һ��
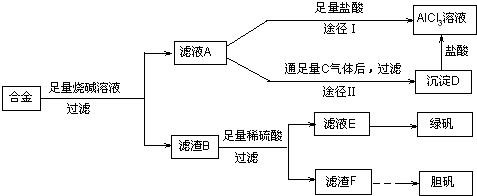
������������Ҫ�����˳��������ʵķ����װ�ã��ѶȲ���������ԭ���ͼ��ˣ�

��ϰ��ϵ�д�
�����Ŀ