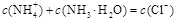��Ŀ����
��10�֣������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
��ش�
(1) �Ӣ������������ HA�� �ᣨѡ�ǿ��������������
(2) �������������c 0.2 (�����������������)��
(3) �Ӣ���ʵ����������˵��HA�ĵ���̶�______NaA��ˮ��̶�(�����������������)���û����Һ������Ũ���ɴ�С��˳���� ��
(4) ����ʵ�����û����Һ����ˮ�������c(OH��)�� mol��L��1��
| ʵ���� | HA���ʵ���Ũ��(mol��L-1) | NaOH���ʵ���Ũ��(mol��L-1) | �����Һ��pH |
| �� | 0.1 | 0.1 | pH��9 |
| �� | c | 0.2 | pH��7 |
| �� | 0.2 | 0.1 | pH��7 |
(1) �Ӣ������������ HA�� �ᣨѡ�ǿ��������������
(2) �������������c 0.2 (�����������������)��
(3) �Ӣ���ʵ����������˵��HA�ĵ���̶�______NaA��ˮ��̶�(�����������������)���û����Һ������Ũ���ɴ�С��˳���� ��
(4) ����ʵ�����û����Һ����ˮ�������c(OH��)�� mol��L��1��
����10�֣�
(1) HA�����(2) ���� (3) �� c(A��) ��c(Na��) ��c(H��)��c(OH��) (4) 10 -5
(1) HA�����(2) ���� (3) �� c(A��) ��c(Na��) ��c(H��)��c(OH��) (4) 10 -5
��

��ϰ��ϵ�д�
�����Ŀ
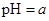 ��������Һϡ��1������Һ��
��������Һϡ��1������Һ��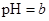 ����
����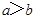
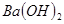 ��Һ�У���μ���һ�����ʵ���Ũ�ȵ�
��Һ�У���μ���һ�����ʵ���Ũ�ȵ�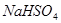 ��Һ������Һ�е�
��Һ������Һ�е�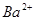 ǡ����ȫ����ʱ����Һ
ǡ����ȫ����ʱ����Һ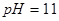 ������Ӧ����Һ���������
������Ӧ����Һ���������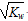 ����Һ
����Һ