��Ŀ����
�ش������й�������ԭ��Ӧ�����⣺
��1�������з�Ӧ�У�A��2F2+2H2O=4HF+O2����
B��2Na+2H2O=2NaOH+H2����
C��CaO+H2O=Ca��OH��2��D��2H2O
2H2��+O2��������ˮֻ������������
��2��ӡˢ��·���������Ϻ�ͭ�����϶��ɣ�����ӡˢ��·ʱҪ��FeCl3��Һ��Ϊ����ʴҺ������CuCl2��FeCl2���䷴Ӧ�Ļ�ѧ����ʽ���£�Cu+2FeCl3�TCuCl2+2FeCl2
������˫���ŷ�����ʽ�б������ת�Ƶ����
���ԱȽ�Fe3+��Cu2+��Fe2+�����Ե�ǿ����
��1�������з�Ӧ�У�A��2F2+2H2O=4HF+O2����
B��2Na+2H2O=2NaOH+H2����
C��CaO+H2O=Ca��OH��2��D��2H2O
| ||
B
B
��ˮֻ�ǻ�ԭ������A
A
��ˮ���������������ǻ�ԭ������D
D
��ˮ�Ȳ������������ֲ��ǻ�ԭ������C
C
������ţ�����2��ӡˢ��·���������Ϻ�ͭ�����϶��ɣ�����ӡˢ��·ʱҪ��FeCl3��Һ��Ϊ����ʴҺ������CuCl2��FeCl2���䷴Ӧ�Ļ�ѧ����ʽ���£�Cu+2FeCl3�TCuCl2+2FeCl2
������˫���ŷ�����ʽ�б������ת�Ƶ����
���ԱȽ�Fe3+��Cu2+��Fe2+�����Ե�ǿ����
Fe3+
Fe3+
��Cu2+
Cu2+
��Fe2+
Fe2+
����������1������Ԫ�ػ��ϼ��Ƿ����仯���Լ��仯�������ж�������������ԭ��Ӧ�еı��ֵ����ʣ�
��2����������ԭ��Ӧ���������õ����ӣ��������ͻ�ԭ��֮���ʧ������Ŀ��ȣ����������������Դ�������������������жϣ�
��2����������ԭ��Ӧ���������õ����ӣ��������ͻ�ԭ��֮���ʧ������Ŀ��ȣ����������������Դ�������������������жϣ�
����⣺��1��2Na+2H2O=2NaOH+H2���У�HԪ�ػ��ϼ۽��ͣ�����ԭ����ˮֻ������������Ӧ2F2+2H2O=4HF+O2���У�OԪ�ػ��ϼ����ߣ���������ˮֻ�ǻ�ԭ������Ӧ2H2O
2H2��+O2���У�H��OԪ�ػ��ϼ۱仯��ˮ���������������ǻ�ԭ������ӦCaO+H2O=Ca��OH��2�У�H��OԪ�ػ��ϼ�û�з����仯��ˮ�Ȳ������������ֲ��ǻ�ԭ����
�ʴ�Ϊ��B��A��D��C��
��2������������ԭ��Ӧ���������õ����ӣ��������ͻ�ԭ��֮���ʧ������Ŀ��ȣ���˫���ŷ��ɱ�ʾΪ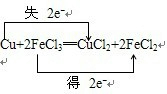 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�ڷ�ӦCu+2Fe2+�TCu2++2Fe2+�У���������Fe3+������������Cu2+���������������Դ�����������������ԣ�����������Fe3+��Cu2+�������Խ�����ǿ�ڽ���ͭ������������Cu2+��Fe2+���ʴ�Ϊ��Fe3+��Cu2+��Fe2+��
| ||
�ʴ�Ϊ��B��A��D��C��
��2������������ԭ��Ӧ���������õ����ӣ��������ͻ�ԭ��֮���ʧ������Ŀ��ȣ���˫���ŷ��ɱ�ʾΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
���ڷ�ӦCu+2Fe2+�TCu2++2Fe2+�У���������Fe3+������������Cu2+���������������Դ�����������������ԣ�����������Fe3+��Cu2+�������Խ�����ǿ�ڽ���ͭ������������Cu2+��Fe2+���ʴ�Ϊ��Fe3+��Cu2+��Fe2+��
������������һ�����ʵ������Ժͻ�ԭ��ǿ���ıȽ�֪ʶ��Ŀ��ע����ɣ�������ԭ��Ӧ�У��������������Դ�����������������ԣ�

��ϰ��ϵ�д�
�����Ŀ



 2H2��+O2��������ˮֻ������������______��ˮֻ�ǻ�ԭ������______��ˮ���������������ǻ�ԭ������______��ˮ�Ȳ������������ֲ��ǻ�ԭ������______������ţ���
2H2��+O2��������ˮֻ������������______��ˮֻ�ǻ�ԭ������______��ˮ���������������ǻ�ԭ������______��ˮ�Ȳ������������ֲ��ǻ�ԭ������______������ţ��� 2H2��+O2��������ˮֻ������������ ��ˮֻ�ǻ�ԭ������ ��ˮ���������������ǻ�ԭ������ ��ˮ�Ȳ������������ֲ��ǻ�ԭ������ ������ţ���
2H2��+O2��������ˮֻ������������ ��ˮֻ�ǻ�ԭ������ ��ˮ���������������ǻ�ԭ������ ��ˮ�Ȳ������������ֲ��ǻ�ԭ������ ������ţ���