��Ŀ����
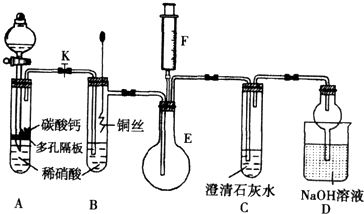
��11�֣� ijУ����С��Ϊ��̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����������ʵ�飬װ����ͼ��ʾ(����װ�ú̶�װ�þ�����ȥ)��ͼ��KΪֹˮ��(���ڹر�״̬)��F��һ��յ�ע������
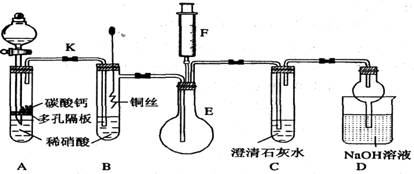
��ش��й����⣺
(1) ���װ��A��Ŀ���� ��
Ϊ�ﵽ��Ŀ�ģ�Ӧ���еIJ����Ǵ�K���Ҵ�Һ©����������װ��C�в���
ʱ���ر�K��
(2) �����(1)�еġ���������װ��B��ͭ˿����ϡ���ᣬ����֮���۲쵽װ��B�е�
������ ��
B�з�Ӧ�����ӷ���ʽΪ�� ��
(3) װ��E��F�������� ��Ϊʵ�ִ����ã������������ ��
(4) װ��D�����������ն���ĵ��������ֹ��Ⱦ���������� �Ĺ��ܡ�
(1)�������ɵĶ�����̼������װ���ڵĿ����Ͼ�������NO��������Ӧ���ɶ����������������Ĺ۲�������š� ��2�֣� ��ɫ������1�֣�
(2)ͭ˿����������ݣ�ϡ����Һ������Ϊ��ɫ����Һ��Ϊ��ɫ��2�֣�
3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��2�֣�
(3)��֤��ɫ����ΪNO����1�֣� ��ע����F�еĿ�������E�л�E�е���ɫ�������뵽ע�����С���2�֣� (4)��ֹ��Һ��������1�֣�
����������1������װ���к��п������ܰ�NO��������NO2���Ӷ�����ʵ�顣������Ҫ�������ɵĶ�����̼������װ���ڵĿ����Ͼ�������NO��������Ӧ���ɶ����������������Ĺ۲�������š������ʯ��ˮ������CO2��������ɫ�������ݴ˿����жϡ�
��2��ϡ������������ԣ��ܰ�ͭ��������������ͭ��NO��ˮ�������������ͭ˿����������ݣ�ϡ����Һ������Ϊ��ɫ����Һ��Ϊ��ɫ���йصķ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��4������NO���ױ���������NO2������EF����������֤��ɫ����ΪNO�ġ�����IJ����ǽ�ע����F�еĿ�������E�л�E�е���ɫ�������뵽ע�����С�
��5��NO2��������ˮ�����Ի��з�ֹ��Һ���������á�

 ��Уͨ��֤��Ч��ҵϵ�д�
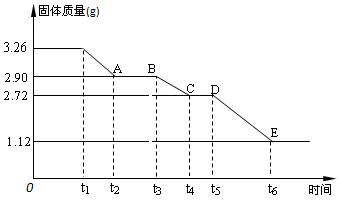
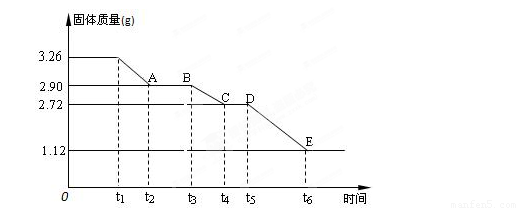
��Уͨ��֤��Ч��ҵϵ�д�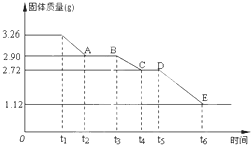 ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4?yH2O����������ʵ�飺��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ������˵��������ǣ�������
ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4?yH2O����������ʵ�飺��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ������˵��������ǣ�������| A��t5��t6ʱ��ι������������ԭ���Dz�����SO2��O2�������� | B��t6��õ��Ĺ�����CaSO4 | C��t2��t3ʱ��ι���Ļ�ѧʽΪ2CaSO4?H2O | D��x��y=2��3 |