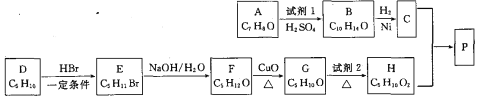
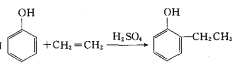��Ŀ����
����Ŀ����1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ��ʪ����ɫ�����Ĺ��ƿ���ɹ۲쵽�������� ____________________��

��2��Ϊ��ֹ����β����Ⱦ���������� __________________ ��Һ���ն����������
��3��Ư���dz��õ���������
�� ��ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ����ʽΪ______________________ ��
�� Ư�۵���Ч�ɷ��ǣ��ѧʽ��__________________________________��
�� Ư������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬ��ѧ����ʽΪ____��
�ܳ���¶���ڿ����е�Ư�ۣ���ϡ���������������� ____________�����ţ���
A��O2 B��Cl2 C��CO2 D��HClO
���𰸡� ������ɫ��������ɫ��ʪ����ɫ������ɫ �������ƣ�NaOH�� 2Cl2+2Ca(OH)2==Ca(ClO)2+CaCl2+2H2O Ca(ClO)2 Ca(ClO)2+CO2+H2O==CaCO3��+2HClO C
�����������������(1).���������û��Ư���ԣ�ʪ�����������Ư���ԣ���2�����������ڼ�����Һ�С�
��3���� ��ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]�����Ȼ��ơ�������ƺ�ˮ��
�� Ư�۵���Ч�ɷ��Ǵ��������
�� Ư������ˮ���ܿ����е�CO2�������ɴ�������̼�����
�ܳ���¶���ڿ����е�Ư����̼������ɡ�
������(1).���������û��Ư���ԣ�ʪ�����������Ư���ԣ����Խ���������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ��ʪ����ɫ�����Ĺ��ƿ���ɹ۲쵽�������Ǹ�����ɫ��������ɫ��ʪ����ɫ������ɫ����2�����������ڼ�����Һ������ֹ����β����Ⱦ��������������������Һ���ն����������
��3���� ��ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]�����Ȼ��ơ�������ƺ�ˮ����Ӧ����ʽΪ2Cl2+2Ca(OH)2==Ca(ClO)2+CaCl2+2H2O��
�� Ư�۵���Ч�ɷ��Ǵ��������
�� Ư������ˮ���ܿ����е�CO2�������ɴ�������̼��Ʒ�Ӧ����ʽΪCa(ClO)2+CO2+H2O==CaCO3��+2HClO��
�ܳ���¶���ڿ����е�Ư����̼�����������ϡ��������CO2������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij����С���ͬѧ����̽��������Һ�¶ȶԱ�ȩ��������Ӧ�������ʵ�Ӱ�졣ʵ���������������£�
1������ʵ��ҩƷ��2������ʵ��������3������������Һ��
4������������Һ�¶ȶԱ�ȩ��������Ӧ�������ʵ�Ӱ��̽��ʵ�顣
��ش��������⣺
��1����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��2��������Ӧѡ��ļ��ȷ����� ��������װ�ñ�ţ���
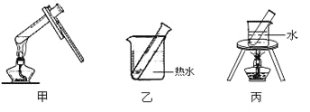
��3������ȤС���ͬѧ̽��������Һ���¶ȶԱ�ȩ��������Ӧ�������ʵ�Ӱ���������±���
��ʵ��ǰ�ⶨ��ʵ���¼������������ʵ�鷽����ֻ�г����¼�������͵�λ��������дʵ�����ݣ���
ʵ����� ʵ����� | ������Һ����/mL | |||
1 | ||||
2 |
��4��ʵ������ϴ�Թ��ڱڸ��ŵ����������ǣ�
��5������Ϊ̽����ȩ����������Ӧ��������������˲ⶨ�������ֵ�ʱ���⣬����Ҫ�Ƚϲ�ͬ�������γɵ������� ��