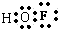��Ŀ����
��������һֱ��Ϊ���ĺ�������ڣ���1971��������ѧ����F2ͨ��ϸ������ôη��ᣨHFO����������HFO���о������˳�����ӡ�
��1���η�������ˮ��Ӧ�õ���ҺA��A�к�B��C�������ʣ�B�����ڵ�̲�����C�ڼ��������·ֽ�����һ����ʹ�����ǵ�ľ����ȼ�����壨��֪2H2O2![]() 2H2O+O2��������η�����ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
2H2O+O2��������η�����ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��д���ڼ���������F2��g�����ռӦ�Ļ�ѧ����ʽ�� ��
��3���жϴη�������Ԫ�صĻ��ϼ� ��
��1��HFO+H2O![]() HF��+H2O2
HF��+H2O2
��2��2F2+4NaOH![]() 4NaF+O2+2H2O
4NaF+O2+2H2O
(3)0��
����:
�����漰���������ԣ���������˼ά�Ĺ��ܡ�ͨ�������£�2F2+2H2O![]() 4HF+O2����Ȼ������Ŀ��Ϣ�ܲ���ͬ����ϸ��������������������ϵ�ġ����Ȱ���Ŀ���������Ϣ������ˮ��Ӧ�ķ���ʽΪ��F2+H2O
4HF+O2����Ȼ������Ŀ��Ϣ�ܲ���ͬ����ϸ��������������������ϵ�ġ����Ȱ���Ŀ���������Ϣ������ˮ��Ӧ�ķ���ʽΪ��F2+H2O![]() HF+HFO������1���С�B�����ڵ�̲���������B��HF����HFO+H2O
HF+HFO������1���С�B�����ڵ�̲���������B��HF����HFO+H2O![]() HF+C��C����Ȼ����F������C�ڼ����·ֽ�����һ����ʹ�����ǵ�ľ����ȼ�����塱����2H2O2
HF+C��C����Ȼ����F������C�ڼ����·ֽ�����һ����ʹ�����ǵ�ľ����ȼ�����塱����2H2O2![]() 2H2O+O2��,��Cֻ����H2O2������η�����ˮ��Ӧ�ķ���ʽΪHFO+H2O
2H2O+O2��,��Cֻ����H2O2������η�����ˮ��Ӧ�ķ���ʽΪHFO+H2O![]() HF��+H2O2�����в��ѿ�����Ӧ2F2+2H2O
HF��+H2O2�����в��ѿ�����Ӧ2F2+2H2O![]() 4HF+O2ʵ�������ڽϸ��¶��µķ�Ӧ�����ڼ���������F2��g�����ռӦʵ������HF��NaOH��Ӧ����HFO�з��Ļ��ϼ�ֻ����-1����Ļ��ϼ�ֻ����+1�������Ļ��ϼ�Ϊ0��
4HF+O2ʵ�������ڽϸ��¶��µķ�Ӧ�����ڼ���������F2��g�����ռӦʵ������HF��NaOH��Ӧ����HFO�з��Ļ��ϼ�ֻ����-1����Ļ��ϼ�ֻ����+1�������Ļ��ϼ�Ϊ0��

| A����+1�ۡ���+1�ۡ���-2�� | B����+1�ۡ���-1�ۡ���0�� | C����-1�ۡ���+3�ۡ���-2�� | D����-1�ۡ���-1�ۡ���+2�� |