��Ŀ����
��������һֱ��Ϊ���ĺ�������ڣ�������1971��˹ͼ�ܶ��Ͱ��������������ɹ��غϳ��˴η���������۵㱻���ҵض�ҡ�ˣ���������0�����½��������ϸ��ĩ������ͨ�����õ��������Ĵη��ᣮ
��1���������ֽṹʽ������ȷ��ʾ�η���ṹ����
��2���η�������Ԫ�صĻ��ϼ�Ϊ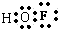
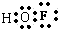 ���η�������й��ۼ��ļ���
���η�������й��ۼ��ļ���
��3����������˼������Ӻͻ��Ż�ѧ���ļ��ܣ�
����㷴Ӧ��2HOF��g��=2HF��g��+O2��g���ķ�Ӧ�ȣ���H��������Ϊ
��4���η����ܱ���ˮ˲��ֽ⣬����һ�ֳ���������M��M��18e-���ұ���Ϊ����ɫ����������д���η�������ˮ��Ӧ�Ļ�ѧ����ʽ��
��1���������ֽṹʽ������ȷ��ʾ�η���ṹ����
A
A
��A��H-O-F B��H-F-O��2���η�������Ԫ�صĻ��ϼ�Ϊ
0
0
���η���ĵ���ʽΪ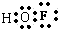
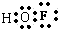
��
��
180�㣨�������=��������3����������˼������Ӻͻ��Ż�ѧ���ļ��ܣ�
| H2 | O2 | F2 | OH | OF | HF | |
| E/��kJ/mol�� | 432 | 494 | 155 | 424 | 220 | 566 |
-338
-338
kJ/mol���Ӽ�����Ԥ��η����һ����ѧ���ʣ�����ǿ������
����ǿ������
����4���η����ܱ���ˮ˲��ֽ⣬����һ�ֳ���������M��M��18e-���ұ���Ϊ����ɫ����������д���η�������ˮ��Ӧ�Ļ�ѧ����ʽ��
HOF+H2O=��=HF+H2O2
HOF+H2O=��=HF+H2O2
����������1���η�����Oԭ��Ϊ����ԭ�ӣ����ݹ��ۼ��ɼ����ص㣬��ԭ���γ�һ�Թ��õ��Ӷԣ���ԭ���γ����Թ��õ��Ӷԣ���ԭ���γ�һ�Թ��õ��Ӷԣ�
��2����ΪFԪ���������ӶԵ�������OԪ��ǿ���ڴη�����FԪ�س�-1�ۣ�����ԭ������������sp3�ӻ���
��3�����ݷ�Ӧ��=��Ӧ����ܼ���-��������ܼ�����������
��4������Ϊ����ɫ���������Ĺ������⣨H2O2����
��2����ΪFԪ���������ӶԵ�������OԪ��ǿ���ڴη�����FԪ�س�-1�ۣ�����ԭ������������sp3�ӻ���
��3�����ݷ�Ӧ��=��Ӧ����ܼ���-��������ܼ�����������
��4������Ϊ����ɫ���������Ĺ������⣨H2O2����
����⣺��1����HFOΪ���ۻ�������ݹ��ۼ��ɼ����ص㣬��ԭ���γ�һ�Թ��õ��Ӷԣ���ԭ���γ����Թ��õ��Ӷԣ���ԭ���γ�һ�Թ��õ��Ӷԣ���ṹʽΪH-O-F���ʴ�Ϊ��A��
��2����ΪFԪ���������ӶԵ�������OԪ��ǿ���ڴη�����FԪ�س�-1�ۣ�HԪ��Ϊ+1�ۣ���OԪ�س���ۣ��η���ĵ���ʽ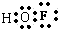 ���η�������ԭ������������sp3�ӻ��������й��ۼ��ļ��ǣ�180�㣬
���η�������ԭ������������sp3�ӻ��������й��ۼ��ļ��ǣ�180�㣬
�ʴ�Ϊ��0��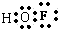 ������
������
��3����Ӧ��=��Ӧ����ܼ���-��������ܼ���=424KJ/mol��2+220 KJ/mol��2-566KJ/mol��2-494KJ/mol=-338KJ/mol���η���ֽ�ų��������ȣ�����ǿ�����ԣ��ʴ�Ϊ��-338������ǿ�����ԣ�
��4������Ϊ����ɫ���������Ĺ������⣨H2O2�����η�������ˮ��Ӧ�Ļ�ѧ����ʽ��HOF+H2O=��=HF+H2O2��
�ʴ�Ϊ��HOF+H2O=��=HF+H2O2��
��2����ΪFԪ���������ӶԵ�������OԪ��ǿ���ڴη�����FԪ�س�-1�ۣ�HԪ��Ϊ+1�ۣ���OԪ�س���ۣ��η���ĵ���ʽ
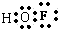 ���η�������ԭ������������sp3�ӻ��������й��ۼ��ļ��ǣ�180�㣬
���η�������ԭ������������sp3�ӻ��������й��ۼ��ļ��ǣ�180�㣬�ʴ�Ϊ��0��
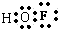 ������
��������3����Ӧ��=��Ӧ����ܼ���-��������ܼ���=424KJ/mol��2+220 KJ/mol��2-566KJ/mol��2-494KJ/mol=-338KJ/mol���η���ֽ�ų��������ȣ�����ǿ�����ԣ��ʴ�Ϊ��-338������ǿ�����ԣ�
��4������Ϊ����ɫ���������Ĺ������⣨H2O2�����η�������ˮ��Ӧ�Ļ�ѧ����ʽ��HOF+H2O=��=HF+H2O2��
�ʴ�Ϊ��HOF+H2O=��=HF+H2O2��
������������һ���ۺ��⣬�������ݽ϶࣬ע��FԪ���������ӶԵ�������OԪ��ǿ���ڴη�����FԪ�س�-1�ۣ�

��ϰ��ϵ�д�
�����Ŀ
��������һֱ��Ϊ���ĺ�������ڣ���1971��������ѧ����F2ͨ��ϸ��ĩ����˴η�����������HFO���о����������ӣ����ж�HFO�и�Ԫ�ػ��ϼ۵�������ȷ���ǣ�������
| A����+1�ۡ���+1�ۡ���-2�� | B����+1�ۡ���-1�ۡ���0�� | C����-1�ۡ���+3�ۡ���-2�� | D����-1�ۡ���-1�ۡ���+2�� |