��Ŀ����
(8��)����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
(1)��֪��N2(g)+O2(g)="2NO(g) " ��H=+180.5kJ/mol
N2(g)+3H2(g )
) 2NH3(g) ��H=��92.4kJ/mol
2NH3(g) ��H=��92.4kJ/mol
2H2(g)+O2(g)=2H2O(g) ��H=��483.6kJ/mol
��ɰ���������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
4NH3��g��+5O2��g��==4NO��g��+6H2O��g������H= kJ��mol
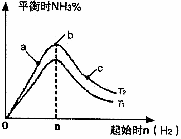
(2)ij����С���о����������������������£��ı���ʼ�����������ʵ����Է�ӦN2(g)+3H2(g)
 2NH3(g)��Ӱ�죮ʵ������ͼ��ʾ��(ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���)
2NH3(g)��Ӱ�죮ʵ������ͼ��ʾ��(ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���)
��ͼ����T1��T2�Ĺ�ϵ�ǣ�T1_______T2(����ڡ������ڡ������ڡ�����ȷ����)
�ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������͵���________(����ĸ)��
(3)��һ���¶Ⱥʹ����£���3.2mol H2��1.2molN2�����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����2minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������0.8mol NH3������������µ�ƽ�ⳣ����(д��������̣��������С�����һλ)
(1)��֪��N2(g)+O2(g)="2NO(g) " ��H=+180.5kJ/mol
N2(g)+3H2(g
 )
) 2NH3(g) ��H=��92.4kJ/mol
2NH3(g) ��H=��92.4kJ/mol2H2(g)+O2(g)=2H2O(g) ��H=��483.6kJ/mol
��ɰ���������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
4NH3��g��+5O2��g��==4NO��g��+6H2O��g������H= kJ��mol

(2)ij����С���о����������������������£��ı���ʼ�����������ʵ����Է�ӦN2(g)+3H2(g)

 2NH3(g)��Ӱ�죮ʵ������ͼ��ʾ��(ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���)
2NH3(g)��Ӱ�죮ʵ������ͼ��ʾ��(ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���)��ͼ����T1��T2�Ĺ�ϵ�ǣ�T1_______T2(����ڡ������ڡ������ڡ�����ȷ����)
�ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������͵���________(����ĸ)��
(3)��һ���¶Ⱥʹ����£���3.2mol H2��1.2molN2�����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����2minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������0.8mol NH3������������µ�ƽ�ⳣ����(д��������̣��������С�����һλ)
��8�֣�
��1����905 ��2�֣�
��2���ٸ��ڣ�1�֣� ��b ��1�֣�
��3��0.4��L2/mol2�������̺ͽ��ȫ�Ե�4�֣���д��λ���۷֣�
��1����905 ��2�֣�
��2���ٸ��ڣ�1�֣� ��b ��1�֣�
��3��0.4��L2/mol2�������̺ͽ��ȫ�Ե�4�֣���д��λ���۷֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2C(g)
2C(g)
 2 NH3��һ�������´ﵽ��ѧ��Ӧ����ʱ�������ж���ȷ����
2 NH3��һ�������´ﵽ��ѧ��Ӧ����ʱ�������ж���ȷ���� 2NH3(g)�ﵽ��ѧƽ��״̬�ı�־��( )
2NH3(g)�ﵽ��ѧƽ��״̬�ı�־��( ) C(g)+D(g) �Ѵﵽ��Ӧ�ȵ���
C(g)+D(g) �Ѵﵽ��Ӧ�ȵ��� Z(g)+2W(g)����ƽ��ʱ��A�����Ϊ1.2 a L ������˵����ȷ����
Z(g)+2W(g)����ƽ��ʱ��A�����Ϊ1.2 a L ������˵����ȷ����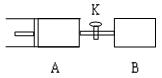
 ��v(A)
��v(A) bZ(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�����˵����ȷ���ǣ� ��
bZ(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�����˵����ȷ���ǣ� ��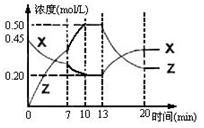
 0��10min�����������ѹǿ������
0��10min�����������ѹǿ������
 N2O4
N2O4