��Ŀ����
ʳƷ��ȫ��ϵ����������Ӱ��ʳƷ��ȫ�����غܶࡣ
(1)��ƫ������ϩ�� ���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������
���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������
_________��д�ṹ��ʽ�������Ӿ۷�Ӧ���ɵġ�
(2)����ֲ�����е�������[CH3(CH2)4-CH=CH-CH2-CH=CH-(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����____��
A������ʽΪC18H34O2 B.һ��������������ͣ�������������������Ӧ
C���ܺ�NaOH��Һ��Ӧ D����ʹ����KMnO4��Һ��ɫ
(3)�پ��м״�(CH3OH)�������꣬��д��Na�ͼ״���Ӧ�Ļ�ѧ����ʽ��___________��
(4)�����̷��е����ʺ����ܵͣ�������ˮ������ղ�����_________��
(5)�ڵ����м�������Ƶõķ�˿�ж����������յ�ˮ������������ǡ������ʵ��֤�������Ѿ�ȫ��ˮ�⣬д����������������ͽ��ۣ�_____________________��
(1)��ƫ������ϩ��
 ���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������
���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������_________��д�ṹ��ʽ�������Ӿ۷�Ӧ���ɵġ�
(2)����ֲ�����е�������[CH3(CH2)4-CH=CH-CH2-CH=CH-(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����____��
A������ʽΪC18H34O2 B.һ��������������ͣ�������������������Ӧ
C���ܺ�NaOH��Һ��Ӧ D����ʹ����KMnO4��Һ��ɫ
(3)�پ��м״�(CH3OH)�������꣬��д��Na�ͼ״���Ӧ�Ļ�ѧ����ʽ��___________��
(4)�����̷��е����ʺ����ܵͣ�������ˮ������ղ�����_________��
(5)�ڵ����м�������Ƶõķ�˿�ж����������յ�ˮ������������ǡ������ʵ��֤�������Ѿ�ȫ��ˮ�⣬д����������������ͽ��ۣ�_____________________��
(1)CCl2=CH2
(2)BCD
(3)2CH3OH+ 2Na��2CH3ONa+H2��
(4)������
(5)ȡ��������ˮ������Һ�������м����ˮ������Һ������֤�������Ѿ�ȫ��ˮ��
(2)BCD
(3)2CH3OH+ 2Na��2CH3ONa+H2��
(4)������
(5)ȡ��������ˮ������Һ�������м����ˮ������Һ������֤�������Ѿ�ȫ��ˮ��

��ϰ��ϵ�д�
�����Ŀ
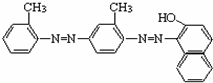 ��2011?˳����ģ�⣩ʳƷ��ȫ��ϵ������������ҵȾ�ϡ��յ���4�š���һ�ַǷ���ʳƷ���Ӽ������Ľṹ��ʽ��ͼ��ʾ�����й��ڡ��յ���4�š�˵���д�����ǣ�������
��2011?˳����ģ�⣩ʳƷ��ȫ��ϵ������������ҵȾ�ϡ��յ���4�š���һ�ַǷ���ʳƷ���Ӽ������Ľṹ��ʽ��ͼ��ʾ�����й��ڡ��յ���4�š�˵���д�����ǣ�������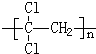 �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������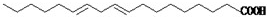 ]�����彡����ʮ������ģ�Ȼ�����г��Ͼ�������ּ۸������ֲ���ͣ����е������Ậ���ܵͣ����й����������˵���в���ȷ����
]�����彡����ʮ������ģ�Ȼ�����г��Ͼ�������ּ۸������ֲ���ͣ����е������Ậ���ܵͣ����й����������˵���в���ȷ����
 �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ������� ����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����£�
����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����£�
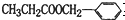 ��Ϊͬ���칹�����
��Ϊͬ���칹�����

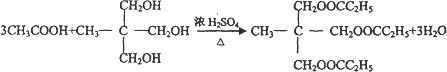
 �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ������� �����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
�����г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������