��Ŀ����
��10�֣�ij�о���ѧϰС����ͨ��ͭ���Ͻ���Ũ����ķ�Ӧ���ⶨ�úϽ����ɡ����ǵ�NO2�ľۺϣ���С��ͬѧ���ĵ��������ϣ�
17�桢1.01��105Pa��2NO2(g) N2O4(g) ��ƽ�ⳣ��K��13.3�����������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱc(NO2)="0.0300" mol��L��1��
N2O4(g) ��ƽ�ⳣ��K��13.3�����������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱc(NO2)="0.0300" mol��L��1��
�Ŵ�ʱƽ����������c(N2O4)�� ��������λ��Ч���֣���ͬ����
��17�桢1.01��105Paʱ����12.44g�Ͻ��������Ũ�����У����Ͻ���ȫ�ܽ���ռ���NO2��N2O4�Ļ������5.00L��
�ٸ���ƽ����ϵ��NO2��N2O4�����ʵ���Ũ�ȣ�����5.00L������NO2��N2O4�����ʵ����ֱ�Ϊ �� ��
�ڼ���Ͻ�����������������
17�桢1.01��105Pa��2NO2(g)
 N2O4(g) ��ƽ�ⳣ��K��13.3�����������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱc(NO2)="0.0300" mol��L��1��
N2O4(g) ��ƽ�ⳣ��K��13.3�����������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱc(NO2)="0.0300" mol��L��1���Ŵ�ʱƽ����������c(N2O4)�� ��������λ��Ч���֣���ͬ����
��17�桢1.01��105Paʱ����12.44g�Ͻ��������Ũ�����У����Ͻ���ȫ�ܽ���ռ���NO2��N2O4�Ļ������5.00L��
�ٸ���ƽ����ϵ��NO2��N2O4�����ʵ���Ũ�ȣ�����5.00L������NO2��N2O4�����ʵ����ֱ�Ϊ �� ��
�ڼ���Ͻ�����������������
��0.0120 mol��L��1�Ƣ�0.15mol 0.06mol
����Ͻ���Cu��Ag�����ʵ����ֱ�Ϊxmol��ymol��
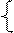 64x+108y��12.44
64x+108y��12.44
2x+y��0.15��0.06��2
��ã�y��0.05mol
m(Ag)��0.05mol��108g/mol��5.4g��3�֣�
w(Ag)��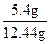 ��100����43.4����1�֣�
��100����43.4����1�֣�
���ڢš��Ƣ���С��ÿ��2�֣��ڢƢ�С��4�֣���10�֣�
����Ͻ���Cu��Ag�����ʵ����ֱ�Ϊxmol��ymol��
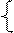 64x+108y��12.44
64x+108y��12.442x+y��0.15��0.06��2
��ã�y��0.05mol
m(Ag)��0.05mol��108g/mol��5.4g��3�֣�
w(Ag)��
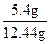 ��100����43.4����1�֣�
��100����43.4����1�֣����ڢš��Ƣ���С��ÿ��2�֣��ڢƢ�С��4�֣���10�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
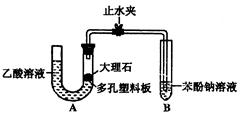
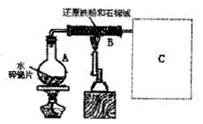
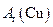 (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������ m(H20)���ɴ˼���
m(H20)���ɴ˼���
 ����_______________ (�����)��
����_______________ (�����)�� ����
���� _____��_______________�ﵽʵ��Ŀ�ġ�
_____��_______________�ﵽʵ��Ŀ�ġ�
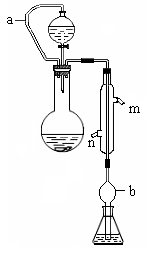
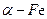 �۾��г�ǿ�Ĵ����ܣ����������ܶȴż�¼�Ľ����Լ���Ч�����ȡ��ڲ�ͬ�¶��£�
�۾��г�ǿ�Ĵ����ܣ����������ܶȴż�¼�Ľ����Լ���Ч�����ȡ��ڲ�ͬ�¶��£�