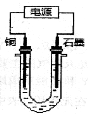
��Ŀ����
����Ŀ���������ԭ�����ش����и�С�⣺
I.��֪��NaHSO4��ˮ�еĵ��뷽��ʽΪNaHSO4=Na����H����SO42�� .
��1�������£�pH��5��NaHSO4��Һ��ˮ�ĵ���̶�______________(�>������������<��)pH��9�İ�ˮ��ˮ�ĵ���̶ȡ�
��2������������ʵ���Ũ�ȵ�NaHSO4�백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ��______________(�����ӷ���ʽ��ʾ)����һ������NaHSO4��Һ�백ˮ��Ϻ���ҺpH��7����c(Na��)��c(NH![]() )______________c(SO42��)(�>������������<��)������������������������Һ��ȡ���ᱵ������Һ��SO42����ȫ��������Ӧ����Һ��pH______________7(�>������������<��)��
)______________c(SO42��)(�>������������<��)������������������������Һ��ȡ���ᱵ������Һ��SO42����ȫ��������Ӧ����Һ��pH______________7(�>������������<��)��
��3�����ֱ���MnO![]() ��Fe3����Fe2����I�� ��������Һ��ϣ�������Һ��pH��ʹpH��1����ַ�Ӧ����I�� ������ʣ�࣬����������������Һ�л����ڵ���______________��һ�������ڵ���______________��
��Fe3����Fe2����I�� ��������Һ��ϣ�������Һ��pH��ʹpH��1����ַ�Ӧ����I�� ������ʣ�࣬����������������Һ�л����ڵ���______________��һ�������ڵ���______________��
II.�ڻ�ѧ�����в���K2CrO4Ϊָʾ������AgNO3����Һ�ζ���Һ��Cl������Ag+��CrO42����ש��ɫ������ָʾ����ζ��յ㡣����Һ��Clǡ�ó�����ȫ(Ũ�ȵ���1.0��105 mol��L1)ʱ����Һ��c(Ag+)Ϊ________________mol��L1����ʱ��Һ��c(CrO42)����______________mol��L1��(��֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ2.0��1012��2.0��1010)��
���𰸡� = NH4++H2O![]() NH3��H2O+H+ > > Fe2�� MnO
NH3��H2O+H+ > > Fe2�� MnO![]() ��Fe3�� 2��10-5 5��10-3
��Fe3�� 2��10-5 5��10-3
����������1��pH=5��NaHSO4��Һ�У�ˮ�ĵ���c��H+��=10-9mol/L��pH=9��NH3H2O��ˮ�ĵ���c��H+��=10-9mol/L��������Һ��ˮ�ĵ���̶���ȣ�
��2������������ʵ���Ũ�ȵ�NaHSO4�백ˮ��Ϻ�Ӧ���������ơ�����泥�笠����Ӳ���ˮ����Һ�����ԣ�ˮ�����ӷ�ӦΪ��NH4++H2ONH3H2O+H+��
pH=7��������������������Ũ����ȣ��ɵ���غ��֪����Ũ�ȹ�ϵΪ��c��Na+��+c��NH4+��=2c��SO42-����c��SO42-��������������������������Һ��ȡ���ᱵ�������������ȫ�������������1��2��Ӧ���������ᱵ��NaOH����Һ�Լ��ԣ���Һ��pH��7��
��3�����������£�����������������Դ��������ӣ������ӻ�ԭ�Դ����������ӣ���ַ�Ӧ����I-������ʣ�࣬����Һ��һ�������ں͵����ӷ�Ӧ��MnO4-��Fe3+���ӣ�����Fe2+��
��4������Һ��Cl-��ȫ����ʱ����c��Cl-��=1.0��10-5mol/L������Ksp��AgCl��=2.0��10-10������õ�c��Ag+��=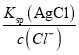 =
=![]() =2.0��10-5mol/L����ʱ��Һ��c��CrO42-��=
=2.0��10-5mol/L����ʱ��Һ��c��CrO42-��=![]() =
= =5.0��10-3mol/L��
=5.0��10-3mol/L��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�