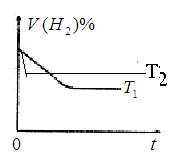
��Ŀ����
(��8��)�ں��º��ݵ��ܱ�������ͨ��1mol N2��Xmol H2�������·�Ӧ��N2��3H2 2NH3���ﵽƽ���÷�Ӧ�ų�������Ϊ18.4kJ�������������ʵ���Ϊ3.6mol�������ڵ�ѹǿ��Ϊԭ����90%��
2NH3���ﵽƽ���÷�Ӧ�ų�������Ϊ18.4kJ�������������ʵ���Ϊ3.6mol�������ڵ�ѹǿ��Ϊԭ����90%��
(1)��ʼʱ����H2�����ʵ���Ϊ mol��������ת����Ϊ ��
(2)�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
(3)����ʼʱ����N2��H2��NH3�����ʵ����ֱ�Ϊa��b��c���ﵽƽ��ʱ��������ʵ���������ƽ����ͬ����ά�ַ�Ӧ����������У�����ʼʱc��ȡֵ��Χ�� ��
 2NH3���ﵽƽ���÷�Ӧ�ų�������Ϊ18.4kJ�������������ʵ���Ϊ3.6mol�������ڵ�ѹǿ��Ϊԭ����90%��
2NH3���ﵽƽ���÷�Ӧ�ų�������Ϊ18.4kJ�������������ʵ���Ϊ3.6mol�������ڵ�ѹǿ��Ϊԭ����90%��(1)��ʼʱ����H2�����ʵ���Ϊ mol��������ת����Ϊ ��
(2)�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
(3)����ʼʱ����N2��H2��NH3�����ʵ����ֱ�Ϊa��b��c���ﵽƽ��ʱ��������ʵ���������ƽ����ͬ����ά�ַ�Ӧ����������У�����ʼʱc��ȡֵ��Χ�� ��
��(1)3�� 20%
(2)N2(g)��3H2(g) 2NH3(g) ��H����92.0kJ/mol (3) 0 mol��c��0.4mol
2NH3(g) ��H����92.0kJ/mol (3) 0 mol��c��0.4mol
(2)N2(g)��3H2(g)
 2NH3(g) ��H����92.0kJ/mol (3) 0 mol��c��0.4mol
2NH3(g) ��H����92.0kJ/mol (3) 0 mol��c��0.4mol��1����Ϊѹǿ֮�������ʵ���֮�ȣ����Է�Ӧǰ��������ʵ�����3.6mol��0.9��4.0mol��������������ʵ�����4mol��1mol��3mol�������ĵ��������ʵ�����amol��������������3amol�����ɰ�����2amol�������1.0mol��amol��3.0mol��3amol��2amol��3.6mol�����a��0.2mol������������ת������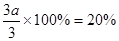 ��
��
��2���ɣ�1����֪����0.2mol�������ų���������18.4kJ���������1mol�����ų�����������18.4kJ��5��92.0kJ�������Ȼ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g) ��H����92.0kJ/mol��
2NH3(g) ��H����92.0kJ/mol��
��3�����ݣ�1����֪��ƽ��ʱ���ɰ�����0.4mol������Ҫʹ��Ӧ����������У�����ʼʱc��ȡֵ��Χ��0 mol��c��0.4mol��
 ��
����2���ɣ�1����֪����0.2mol�������ų���������18.4kJ���������1mol�����ų�����������18.4kJ��5��92.0kJ�������Ȼ�ѧ����ʽΪN2(g)��3H2(g)
 2NH3(g) ��H����92.0kJ/mol��
2NH3(g) ��H����92.0kJ/mol����3�����ݣ�1����֪��ƽ��ʱ���ɰ�����0.4mol������Ҫʹ��Ӧ����������У�����ʼʱc��ȡֵ��Χ��0 mol��c��0.4mol��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2/3Fe(s)+CO2(g)
2/3Fe(s)+CO2(g) bB(��) + cC(��), ���¶Ȳ����������,�ٳ���һ������A����, ���´ﵽƽ��ʱ, �����ж�����ȷ����:��������
bB(��) + cC(��), ���¶Ȳ����������,�ٳ���һ������A����, ���´ﵽƽ��ʱ, �����ж�����ȷ����:��������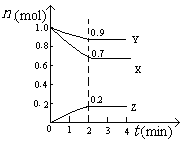
 2C(g)�ﵽ��ѧƽ��״̬�ı�־��
2C(g)�ﵽ��ѧƽ��״̬�ı�־��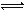 Z(g)����60s�ﵽƽ�⣬����0.3 mol Z��������ȷ����( )
Z(g)����60s�ﵽƽ�⣬����0.3 mol Z��������ȷ����( )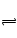 3C(g)���ﵽƽ�������1.5 mol C����ʱ��ƽ��������C���������Ϊ�أ������¶����ߵ�70����������������䣬����Ӧ���´ﵽƽ��ʱ��C�����ʵ���Ϊ1.2 mol��
3C(g)���ﵽƽ�������1.5 mol C����ʱ��ƽ��������C���������Ϊ�أ������¶����ߵ�70����������������䣬����Ӧ���´ﵽƽ��ʱ��C�����ʵ���Ϊ1.2 mol�� 2NH3(g��;
2NH3(g��;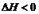 ,10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3�����ʵ���Ϊ0.4mol��
,10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3�����ʵ���Ϊ0.4mol��