��Ŀ����
��25ml 0.1mol��L-1NaOH��Һ����μ���0.2mol��L-1CH3COOH��Һ����ҺpH�仯������ͼ��ʾ�������й�����Ũ�ȵıȽ���ȷ����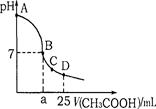
A����A��B����һ��(����A��B��)����Һ�п�����C(Na+) > C(CH3COO��) > C(OH��) > C(H+)
B����B�㣬a>12.5������C(Na+) = C(CH3COO��) = C(OH��) = C(H+)
C����C�㣬C(CH3COO��) > C(Na+) > C(OH��) > C(H+)
D����D�㣬C(CH3COO��) + C(CH3COOH) = C(Na+)
A
���������������A��B����һ�㣬��Һ��ֻ��������������Na+��H+��CH3COO-��OH-�����ݵ���غ����У�c��Na+��+c��H+����c��CH3COO-��+c��OH-����c��H+����c��OH-������Һ������Ũ�ȴ�С��ϵΪ��c��Na+����c��CH3COO-����c��OH-����c��H+������A��ȷ����B����Һ�����ԣ�������c��OH-����c��H+�������ݵ���غ�c��Na+��+c��H+����c��CH3COO-��+c��OH-������һ����c��Na+����c��CH3COO-������Һ�ijɷ�Ϊ����Ӧ���ɵĴ����ƺ�ʣ��Ĵ��ᣬ�����Ƶ�ˮ��̶Ⱥʹ���ĵ���̶���ȣ����У�c��Na+��=c��CH3COO-����c��OH-��=c��H+������B������C�㣬��Һ�����ԣ�����c��OH-����c��H+�������ݵ���غ㣺c��Na+��+c��H+����c��CH3COO-��+c��OH-������c��Na+����c��CH3COO-����c��CH3COO-����c��Na+����c��H+����c��OH-������C������D��ʱ������ʣ�࣬ʣ��Ĵ����Ũ�Ⱥ����ɵĴ�����Ũ����Ⱦ�Ϊ0.05mol/l�����������غ㣬��c��CH3COO-��+c��CH3COOH����0.1mol?L-1��c��Na+��=0..05mol/L��c��CH3COO-��+c ��CH3COOH��=2c��Na+����D����ѡA��
���㣺��������к�ͼ���������Һ������Ũ�ȴ�С�Ƚϵ�
�����������Ǹ߿��еij������ͣ������ۺ�������Ŀ��飬��ѧ����˼ά��������˽ϸߵ�Ҫ���������߿����ۺ���ǿ�����ض�ѧ����������������������ѧ���������������ʹ���˼ά������������һ������Ϻ�����Ũ�ȴ�С�Ƚϵ���Ŀ��Ҫ�����ÿ��״̬ʱ��Һ������������ע������غ�˼���������á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��й�������Һ��������ȷ����
| A������������ƵĻ����Һ�У�c(CH3COO��)+ c(OH��) = c(Na+) + c(H+) |
| B��1 mol��L��1Na2CO3��Һ�У�c(OH��) = c((H+) + c(HCO3��) + c(H2CO3) |
| C��0.1 mol��L��1���������Һ�У�c(SO42��) >c(NH4+)>c(H+)> c(OH��) |
| D����1molKCl��1molNaHS����ˮ���1L�����Һ�У� |
����������������Һ���й�������ȷ����
| ��� | �� | �� |
| pH | 12 | 2 |
| ��Һ | ����������Һ | ������Һ |
A��������Һ��ˮ�ĵ���̶ȣ���=��
B��������Һ��Ũ�ȣ�c(NaOH)��c(CH3COOH)
C��������Һ�������Ϻ�c(CH3COO-)��c(Na+)��c(OH-)��c(H+)
D����ˮϡ����ͬ������c(Na+)��c(CH3COO-)
�����Ƕ�Ԫ���ᣬ���������Һ�����ԡ���O.1mol��L��1 KHC2O4��Һ�У����й�ϵ��ȷ���ǣ� ��
| A��c(K+)+c(H+) = c(HC2O4��)+c(OH��)+c(C2O42��) |
| B��c(HC2O4��)+c(C2O42��) =" 0.1" mol��L��1 |
| C��c(C2O42��) < c(H2C2O4) |
| D��c(K+) = c(H2C2O4)+c(HC2O4��)+c(C2O42��) |
����������ȷ���� �� ��
| A��0.1 mol��L-1CH3COOH��Һ��ˮϡ�ͣ���Һ����������Ũ�Ⱦ���С |
| B�������£�����ˮ�������c(OH-)=1��10-12mol?L-1����Һ�У�Al3+���ܴ������� |
| C�������£���AgCl����Һ�м�������NaCl�������Ksp��AgCl������ |
| D�������£���ͬŨ�ȵ�CH3COONa��Һ��Na2CO3��Һ��ȣ�Na2CO3��Һ��pHС |
��֪25��ʱ��AgCl���ܶȻ�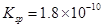 ��������˵����ȷ���ǣ�
��������˵����ȷ���ǣ�
| A����AgClˮ��Һ�м������ᣬKspֵ��� |
B��AgNO3��Һ��NaCl��Һ��Ϻ����Һ�У�һ���� |
C���¶�һ��ʱ������Һ��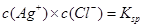 ʱ������Һ�б���AgCl�ij������� ʱ������Һ�б���AgCl�ij������� |
| D����AgCl���뵽��Ũ��KI��Һ�У�����AgClת��ΪAgI����ΪAgCl�ܽ�ȴ���AgI |
10��ʱ���ȱ���NaHCO3��Һ����ø���Һ��pH���������ʾ�ı仯��
| �¶�/�� | 10 | 20 | 30 | ������к���ȴ��50�� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
��ͬѧ��Ϊ������ҺpH�����ԭ����HCO
 ��ˮ��̶����ʼ�����ǿ���йط�Ӧ�����ӷ���ʽΪ__________________________����ͬѧ��Ϊ����ҺpH�����ԭ����NaHCO3���ȷֽ�������Na2CO3�����ƶ�Na2CO3��ˮ��̶�________(����ڡ���С�ڡ�)NaHCO3��ˮ��̶ȣ��÷ֽⷴӦ�Ļ�ѧ����ʽΪ______________________________��
��ˮ��̶����ʼ�����ǿ���йط�Ӧ�����ӷ���ʽΪ__________________________����ͬѧ��Ϊ����ҺpH�����ԭ����NaHCO3���ȷֽ�������Na2CO3�����ƶ�Na2CO3��ˮ��̶�________(����ڡ���С�ڡ�)NaHCO3��ˮ��̶ȣ��÷ֽⷴӦ�Ļ�ѧ����ʽΪ______________________________����ͬѧ��Ϊ���ס��ҵ��ж϶�����֣�����������̽������֤���ǵ��ж��Ƿ���ȷ��
(1)�ڼ�����е���Һ�м����������Լ�X����������������________(��ס����ҡ�)���ж���ȷ���Լ�X��________(�����)��
A��Ba(OH)2��Һ B��BaCl2��Һ
C��NaOH��Һ D������ʯ��ˮ
(2)�����Ⱥ����Һ��ȴ��10�棬����Һ��pH________(����ڡ���С�ڡ����ڡ�)8.3����________(��ס����ҡ�)���ж���ȷ��
(3)��ͬѧ�������Ϻ��֣�NaHCO3�ķֽ��¶�Ϊ150�棬������________(��ס����ҡ�)���ж��Ǵ���ģ�ԭ����________________________________________________��
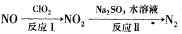
 ��
�� ��
��
 ___pH
___pH ������
������