��Ŀ����
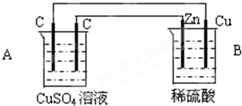
 ��ͼװ���У�A��B������Һ�������Ϊ200mL��
��ͼװ���У�A��B������Һ�������Ϊ200mL����1���ж�װ�õ����ƣ�A��Ϊ
����
����
B��Ϊԭ���
ԭ���
��2��A�������ʯī��Ϊ
��
��
�����缫��ӦʽΪ4OH--4e-=2H2O+O2��
4OH--4e-=2H2O+O2��
A���е���ܷ�Ӧʽ
2H2O+2CuSO4=2Cu+O2��+2H2SO4
2H2O+2CuSO4=2Cu+O2��+2H2SO4
��3��������һ��ʱ���B����Cu��������224ml���壨��״̬�������·��ͨ����
0.02
0.02
mol���ӣ�B������Һ��������
����
������ӡ��������䡱���١���0.63
0.63
g������Ӧǰ����Һ��������䣬��Ӧ��A����Һ��pHΪ1
1
��������Bװ�����Է��Ľ���������ԭ��Ӧ������B��ԭ��أ���A�ǵ��أ�B��п��ʧ������������ͭ����������A�����̼�����������ұ�̼�������������������������ӷŵ磬������ͭ���ӷŵ磬����ͭ��ת�Ƶ��ӵĹ�ϵ�����йؼ��㣮
����⣺��1��Bװ�����Է��Ľ���������ԭ��Ӧ������B��ԭ��أ���A�ǵ��أ�
�ʴ�Ϊ�����أ�ԭ��أ�
��2��B��п��������ͭ����������A�����̼�����������ұ�̼�������������������������ӷŵ������������缫��ӦʽΪ��4OH--4e-=2H2O+O2����������ͭ���ӷŵ�����ͭ�����Ե�ط�ӦʽΪ��2H2O+2CuSO4=2Cu+O2��+2H2SO4��
�ʴ�Ϊ������4OH--4e-=2H2O+O2����2H2O+2CuSO4=2Cu+O2��+2H2SO4��
��3��B��ͭ���ϵ缫��ӦʽΪ��2H++2e-=H2�����ݵ缫��Ӧʽ֪��ת�Ƶ��ӵ����ʵ���=
��2=0.02mol��B���н������п���ӣ���������������ת����ͬ�ĵ���ʱ��������������ڳ�����������������Һ�������ӣ����ӵ�����=
��(65-3)g/mol=0.63g��2H2O+2CuSO4=2Cu+O2��+2H2SO4 ֪����ת��0.02mol����ʱ�����ɵ������������ӵ����ʵ���=
��2��2=0.02mol����������Ũ��=
=0.1mol/L��������ph=1��
�ʴ�Ϊ��0.02�����ӣ�0.63��1��
�ʴ�Ϊ�����أ�ԭ��أ�
��2��B��п��������ͭ����������A�����̼�����������ұ�̼�������������������������ӷŵ������������缫��ӦʽΪ��4OH--4e-=2H2O+O2����������ͭ���ӷŵ�����ͭ�����Ե�ط�ӦʽΪ��2H2O+2CuSO4=2Cu+O2��+2H2SO4��
�ʴ�Ϊ������4OH--4e-=2H2O+O2����2H2O+2CuSO4=2Cu+O2��+2H2SO4��
��3��B��ͭ���ϵ缫��ӦʽΪ��2H++2e-=H2�����ݵ缫��Ӧʽ֪��ת�Ƶ��ӵ����ʵ���=
| 0.224L |
| 22.4L/mol |
| 0.224L |
| 22.4L/mol |
| 0.02mol |
| 4 |
| 0.02mol |
| 0.2L |
�ʴ�Ϊ��0.02�����ӣ�0.63��1��
���������⿼����ԭ��غ͵���ԭ������ȷԭ�������������������������ж��ǽⱾ��ؼ����ѵ��Ǽ�����Һ��pH���Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ