��Ŀ����
���ᡢ�������������ѧ�γ����ġ������ᡱ����͡������ᡱ�����ͭ��Ӧ��������ش��������⣺(1)����֪����ϡ������ͭ����Ӧ������ϡ�����м���H2O2�����ʹͭ˳���ܽ⡣�÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
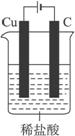
ijͬѧδ���������������������һ��ʵ��װ�ã�Ҳ��ʹͭ����ϡ���ᡣ�������з����л�����װ��ͼ��

(2)��һ�������18 mol��L-1��Ũ�����м��������ͭƬ������ʹ֮��Ӧ������ԭ��������0.9 mol����������ʵ�����____________(����ڡ������ڡ���С�ڡ�)100 mL������ʹʣ���ͭƬ�����ܽ⣬�������м��������Σ��÷�Ӧ�����ӷ���ʽΪ______________________��
(3)�����ݵ�������ͭƬ�ֱ���������������Ũ�����ϡ���ᷴӦ�����õ�����Һǰ�߳���ɫ�����߳���ɫ��ijͬѧ����������Cu2+Ũ�Ȳ�ͬ����ģ���ͬ�����ֿ�����?__________(�ͬ�⡱��ͬ�⡱)��ԭ����____________________________________����һͬѧ�����Һ�ʡ���ɫ������Һ��Cu2+��NO2����Ľ�����������ʵ��̽����˵����ȷ���(����ʵ�鷽����ʵ�������ɴ˵ó��Ľ���)____________________________________��
(1)Cu+H2O2+2HCl![]() CuCl2+2H2O
CuCl2+2H2O
(2)���� 3Cu+8H++2![]()
![]() 3Cu2++2NO��+4H2O��Cu+4H++2
3Cu2++2NO��+4H2O��Cu+4H++2![]()
![]() Cu2++ 2NO2��+2H2O��д�������ӷ���ʽ
Cu2++ 2NO2��+2H2O��д�������ӷ���ʽ
(3)��ͬ�� ��������ͭƬ��������������Ũ�����ϡ���ᷴӦ��������Һ��Cu2+��Ũ�Ȼ������ ��һ������NO2ͨ��ͭƬ��ϡ���ᷴӦ�����Һ�У�����Һ����ɫ�����ͬѧ�Ľ�����ȷ����֮������ȷ��(��ͭƬ��Ũ���ᷴӦ�����Һ��������ˮϡ�ͣ�����Һ��Ϊ��ɫ�����ͬѧ�Ľ�����ȷ����֮������ȷ)
���������������Ҫע������������ͭ��Ӧ��������������ͭ��Ӧ������˫��ˮ�������ǽ�����ͭ����ΪCuO��Ȼ������ͭ���������Ҫ���ʵ�飬ʹ����ͭ�������ᣬ�����ԭ��أ������ͭ������������������ʴ������һ���̱Ƚϻ�����Ҳ������Ƶ��أ������ͭ���������Ӷ��ó�ʵ��ͼ������(2)Ҫע��Ũ����ͽ���ͭ��Ӧ�Ĺ�����Ũ�������������ã�һ��������������һ������ʾ���ԣ����������������100 mL��

 ���ᡢ�������������ѧ�γ����ġ������ᡱ���־������������ͭ��Ӧ��������ش��������⣺
���ᡢ�������������ѧ�γ����ġ������ᡱ���־������������ͭ��Ӧ��������ش��������⣺

