ΧβΡΩΡΎ»ί
| ‘ΣΥΊ | H | Li | Be | B | C | N | O | F |
| ΒγΗΚ–‘ | 2.1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| ‘ΣΥΊ | Na | Mg | Al | Si | P | S | Cl | K |
| ΒγΗΚ–‘ | 0.9 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 | 3.0 | 0.8 |
Θ®1Θ©‘Λ≤β÷ήΤΎ±μ÷–ΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊ”ΠΈΣ
Θ®2Θ©ΗυΨί±μ÷–ΒΡΥυΗχ ΐΨίΖ÷ΈωΘ§Ά§÷ςΉεΡΎΒΡ≤ΜΆ§‘ΣΥΊXΒΡ÷Β±δΜ·
Θ®3Θ©Ψ≠―ιΙφ¬…ΗφΥΏΈ“Ο«ΘΚΒ±–Έ≥…Μ·―ßΦϋΒΡΝΫ‘≠Ή”œύ”Π‘ΣΥΊΒΡΒγΗΚ–‘≤ν÷Β¥σ”Ύ1.7 ±Θ§Υυ–Έ≥…ΒΡ“ΜΑψΈΣάκΉ”ΦϋΘΜΒ±–Γ”Ύ1.7 ±Θ§“ΜΑψΈΣΙ≤ΦέΦϋΘ° ‘ΆΤΕœAlBr3÷––Έ≥…ΒΡΜ·―ßΦϋΒΡάύ–ΆΈΣ
”…±μ÷– ΐΨίΩ…÷ΣΘ§Ά§÷ήΤΎΉ‘ΉσΕχ”“ΒγΗΚ–‘‘ω¥σΘ§Ά§÷ςΉεΉ‘…œΕχœ¬ΒγΗΚ–‘ΫΒΒΆΘ§Ζ«Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ«ΩΘ§Ι Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ–ΓΘ§ΗΤ‘ΣΥΊΒΡΒγΗΚ–‘±»K‘ΣΥΊ¥σΘ§ΒΪ–Γ”ΎLi‘ΣΥΊΒΡΒγΗΚ–‘ΘΜ
Θ®2Θ©”…±μ÷– ΐΨίΩ…÷ΣΘ§Ά§÷ήΤΎΉ‘ΉσΕχ”“ΒγΗΚ–‘‘ω¥σΘ§Ά§÷ςΉεΉ‘…œΕχœ¬ΒγΗΚ–‘ΫΒΒΆΘ§Ι Ζ«Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ¥σΘ§Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ–ΓΘΜ
Θ®3Θ©AlCl3÷–ΝΫΒγΗΚ–‘÷°≤νΈΣ1.5Θ§Br‘ΣΥΊΒΡΒγΗΚ–‘–Γ”ΎCl‘ΣΥΊΒγΗΚ–‘Θ§AlBr3÷–ΝΫΒγΗΚ–‘÷°≤ν–Γ”Ύ1.5Θ°
”…±μ÷– ΐΨίΩ…÷ΣΘ§Ά§÷ήΤΎΉ‘ΉσΕχ”“ΒγΗΚ–‘‘ω¥σΘ§Ά§÷ςΉεΉ‘…œΕχœ¬ΒγΗΚ–‘ΫΒΒΆΘ§Ζ«Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ«ΩΘ§Ι Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ–ΓΘ§Ι ΗΤ‘ΣΥΊΒΡΒγΗΚ–‘±»K‘ΣΥΊ¥σΘ§ΒΪ–Γ”ΎLi‘ΣΥΊΒΡΒγΗΚ–‘Θ§Φ¥0.8ΘΦXΘ®CaΘ©ΘΦ1Θ§
Ι ¥πΑΗΈΣΘΚFΘ§0.8ΓΔ1ΘΜ
Θ®2Θ©”…±μ÷– ΐΨίΩ…÷ΣΘ§Ά§÷ήΤΎΉ‘ΉσΕχ”“ΒγΗΚ–‘‘ω¥σΘ§Ά§÷ςΉεΉ‘…œΕχœ¬ΒγΗΚ–‘ΫΒΒΆΘ§Ι Ζ«Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ¥σΘ§Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ–ΓΘ§
Ι ¥πΑΗΈΣΘΚΉ‘…œΕχœ¬ΒγΗΚ–‘ΫΒΒΆΘ§Ζ«Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ¥σΘ§Ϋπ τ–‘‘Ϋ«ΩΒγΗΚ–‘‘Ϋ–ΓΘΜ
Θ®3Θ©AlCl3÷–ΝΫΒγΗΚ–‘÷°≤νΈΣ1.5Θ§Br‘ΣΥΊΒΡΒγΗΚ–‘–Γ”ΎCl‘ΣΥΊΒγΗΚ–‘Θ§AlBr3÷–ΝΫΒγΗΚ–‘÷°≤ν–Γ”Ύ1.5Θ§Ι AlBr3÷–Μ·―ßΦϋΈΣΙ≤ΦέΦϋΘ§
Ι ¥πΑΗΈΣΘΚΙ≤ΦέΦϋΘ§AlCl3÷–ΝΫΒγΗΚ–‘÷°≤νΈΣ1.5Θ§Br‘ΣΥΊΒΡΒγΗΚ–‘–Γ”ΎCl‘ΣΥΊΒγΗΚ–‘Θ§AlBr3÷–ΝΫΒγΗΚ–‘÷°≤ν–Γ”Ύ1.5Θ°

‘ΣΥΊ | H | Li | Be | B | C | N | O | F |
ΒγΗΚ–‘ | 2.1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
‘ΣΥΊ | Na | Mg | Al | Si | P | S | Cl | K |
ΒγΗΚ–‘ | 0.9 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 | 3.0 | 0.8 |
«κΉ–œΗΖ÷ΈωΘ§Άξ≥…œ¬Ν–”–ΙΊΈ ΧβΘΚ
Θ®1Θ©‘Λ≤β÷ήΤΎ±μ÷–ΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊ”ΠΈΣ______ΘΜΙάΦΤΗΤ‘ΣΥΊΒΡΒγΗΚ–‘ΒΡ»Γ÷ΒΖΕΈßΘΚ__________ΘΦXΘΦ__________ΓΘ
Θ®2Θ©ΗυΨί±μ÷–ΒΡΥυΗχ ΐΨίΖ÷ΈωΘ§Ά§÷ςΉεΡΎΒΡ≤ΜΆ§‘ΣΥΊXΒΡ÷Β±δΜ·ΒΡΙφ¬… «_______________________________________ΘΜΦρ ω‘ΣΥΊΒγΗΚ–‘XΒΡ¥σ–Γ”κ‘ΣΥΊΫπ τ–‘ΓΔΖ«Ϋπ τ–‘÷°ΦδΒΡΙΊœΒ________________________________________ΓΘ?
Θ®3Θ©Ψ≠―ιΙφ¬…ΗφΥΏΈ“Ο«ΘΚΒ±–Έ≥…Μ·―ßΦϋΒΡΝΫ‘≠Ή”œύ”Π‘ΣΥΊΒΡΒγΗΚ–‘≤ν÷Β¥σ”Ύ1.7 ±Θ§Υυ–Έ≥…ΒΡ“ΜΑψΈΣάκΉ”ΦϋΘΜΒ±–Γ”Ύ1.7 ±Θ§“ΜΑψΈΣΙ≤ΦέΦϋΓΘ ‘ΆΤΕœAlBr3÷––Έ≥…ΒΡΜ·―ßΦϋΒΡάύ–ΆΈΣ__________Θ§Τδάμ”… «__________ΓΘ
Θ®10Ζ÷Θ©1932ΡξΟάΙζΜ·―ßΦ“±ΪΝ÷ Ήœ»Χα≥ωΝΥΒγΗΚ–‘ΒΡΗ≈ΡνΓΘΒγΗΚ–‘(”ΟX±μ Ψ)“≤ «‘ΣΥΊΒΡ“Μ÷÷÷Ί“Σ–‘÷ Θ§œ¬±μΗχ≥ωΒΡ «‘≠Ή”–ρ ΐ–Γ”Ύ20ΒΡ16÷÷‘ΣΥΊΒΡΒγΗΚ–‘ ΐ÷ΒΘΚ
| ‘ΣΥΊ | H | Li | Be | B | C | N | O | F |
| ΒγΗΚ–‘ | 2.1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| ‘ΣΥΊ | Na | Mg | Al | Si | P | S | Cl | K |
| ΒγΗΚ–‘ | 0.9 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 | 3.0 | 0.8 |
ΔΌ ‘Λ≤β÷ήΤΎ±μ÷–ΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊ”ΠΈΣ____________ΘΜΙάΦΤΗΤ‘ΣΥΊΒΡΒγΗΚ–‘ΒΡ»Γ÷ΒΖΕΈßΘΚ0.8ΘΦ X ΘΦ___________ΓΘ
ΔΎ–¥≥ωKΒΡΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΘΚ
KΘΚ_______________________________________
ΔέΗυΨί±μ÷–ΒΡΥυΗχ ΐΨίΖ÷ΈωΘ§Ά§÷ςΉεΡΎΒΡ≤ΜΆ§‘ΣΥΊXΒΡ÷Β±δΜ·ΒΡΙφ¬…
« ΘΜ Φρ ω‘ΣΥΊΒγΗΚ–‘XΒΡ¥σ–Γ”κ‘ΣΥΊΫπ τ–‘ΓΔΖ«Ϋπ τ–‘÷°ΦδΒΡΙΊœΒ____________________ΓΘ
Θ®11Ζ÷Θ©
Θ®1Θ©ΓΔœ¬±μ «“Μ–©ΕΧ÷ήΤΎ‘ΣΥΊΒΡΤχΧ§‘≠Ή” ß»ΞΚΥΆβ≤ΜΆ§ΒγΉ”Υυ–ηΒΡΡήΝΩ(kJΓΛmol-1)ΘΚ
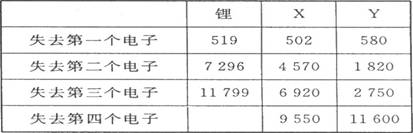
Ά®Ιΐ±μ÷–ΒΡ ΐΨίΖ÷ΈωΈΣ ≤Ο¥ο°‘≠Ή” ß»ΞΚΥΆβΒΎΕΰΗωΒγΉ” ±Υυ–ηΒΡΡήΝΩ“Σ‘Ε‘Ε¥σ”Ύ ß»ΞΒΎ“ΜΗωΒγΉ”Υυ–ηΒΡΡήΝΩ Θ§
X‘Ύ÷ήΤΎ±μ÷–ΈΜ÷ΟΘΚΒΎ ΤΎΘ§ ΉεΘ§YΒΡΉνΗΏ’ΐΦέΈΣ ΓΘ
Θ®2Θ©1932ΡξΟάΙζΜ·―ßΦ“±ΪΝ÷Θ®LΘ°PaulingΘ© Ήœ»Χα≥ωΝΥΒγΗΚ–‘ΒΡΗ≈ΡνΓΘΒγΗΚ–‘Θ®”ΟX±μ ΨΘ©“≤ «‘ΣΥΊΒΡ“Μ÷÷÷Ί“Σ–‘÷ Θ§œ¬±μΗχ≥ωΒΡ «‘≠Ή”–ρ ΐ–Γ”Ύ20ΒΡ16÷÷‘ΣΥΊΒΡΒγΗΚ–‘ ΐ÷ΒΘΚ
|
‘ΣΥΊ |
H |
Li |
Be |
B |
C |
N |
O |
F |
|
ΒγΗΚ–‘ |
2.1 |
1.0 |
1.5] |
2.0 |
2.5 |
3.0 |
3.5 |
4.0 |
|
‘ΣΥΊ |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
K |
|
ΒγΗΚ–‘ |
0.9 |
1.2 |
1.5 |
1.7 |
2.1 |
2.3 |
3.0 |
0.8 |
«κΉ–œΗΖ÷ΈωΘ§ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
ΔΌΓΔ…œ±μ÷–ΒγΗΚ–‘Ήν–ΓΒΡ‘ΣΥΊ « (Χν‘ΣΥΊΖϊΚ≈)Θ§ΙάΦΤΗΤ‘ΣΥΊΒΡΒγΗΚ–‘ΒΡ»Γ÷ΒΖΕΈßΘΚ__________ΘΦXΘΦ__________ΓΘ
ΔΎΓΔΨ≠―ιΙφ¬…ΗφΥΏΈ“Ο«ΘΚΒ±–Έ≥…Μ·―ßΦϋΒΡΝΫ‘≠Ή”œύ”Π‘ΣΥΊΒΡΒγΗΚ–‘≤ν÷Β¥σ”Ύ1.7 ±Θ§Υυ–Έ≥… ΒΡ“ΜΑψΈΣάκΉ”ΦϋΘΜΒ±–Γ”Ύ1.7 ±Θ§“ΜΑψΈΣΙ≤ΦέΦϋΓΘ ‘ΆΤΕœAlBr3÷––Έ≥…ΒΡΜ·―ßΦϋΒΡάύ–ΆΈΣ
______________Θ§Τδάμ”… « ΓΘ