��Ŀ����
���ݰ�����ԭ����ͭ�ķ�Ӧ������ƲⶨͭԪ�����ԭ������Ar(Cu)(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H2O)���ɴ˼���Ar (Cu)��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4Cl��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
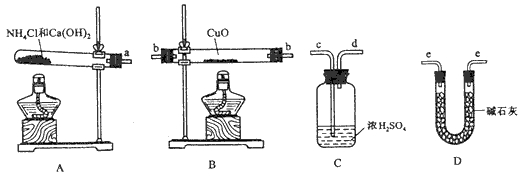
��ش��������⣺
(1)������ԭ��������ͭ�Ļ�ѧ����ʽΪ____________________________��
(2)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a��__________________��
(3)�ڱ�ʵ���У������m(CuO)= a g��m(H2O)= b g����Ar(Cu)= _______________��
(4)�ڱ�ʵ���У�ʹ�ⶨ���Ar(Cu)ƫ�����_______________ (�����)��
��CuOδ��ȫ��Ӧ
�� CuO������
��CuO�л��в���Ӧ������
�� ��ʯ�Ҳ�����
��NH4C1��Ca(OH)2����ﲻ����
(5)�ڱ�ʵ���У�����ͨ���ⶨ______��_____����_____��______�ﵽʵ��Ŀ�ġ�
(1)������ԭ��������ͭ�Ļ�ѧ����ʽΪ____________________________��
(2)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a��__________________��
(3)�ڱ�ʵ���У������m(CuO)= a g��m(H2O)= b g����Ar(Cu)= _______________��
(4)�ڱ�ʵ���У�ʹ�ⶨ���Ar(Cu)ƫ�����_______________ (�����)��
��CuOδ��ȫ��Ӧ
�� CuO������
��CuO�л��в���Ӧ������
�� ��ʯ�Ҳ�����
��NH4C1��Ca(OH)2����ﲻ����
(5)�ڱ�ʵ���У�����ͨ���ⶨ______��_____����_____��______�ﵽʵ��Ŀ�ġ�
(1)2NH3+3CuO 3Cu+3H2O+N2��
3Cu+3H2O+N2��
(2)a��e��b��e��e
(3)18a/b-16
(4) �٢�
(5) m(CuO)��m(Cu)�� m(Cu)��m(H2O)
 3Cu+3H2O+N2��
3Cu+3H2O+N2��(2)a��e��b��e��e
(3)18a/b-16
(4) �٢�
(5) m(CuO)��m(Cu)�� m(Cu)��m(H2O)

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼��� (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������ m(H20)���ɴ˼���
m(H20)���ɴ˼���
 ����_______________ (�����)��
����_______________ (�����)�� ����
���� _____��_______________�ﵽʵ��Ŀ�ġ�
_____��_______________�ﵽʵ��Ŀ�ġ� (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���
