��Ŀ����
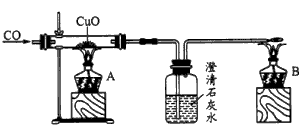
��ͼ�Ǽ�ͬѧ��Ƶ�֤��CO���л�ԭ�Ե�ʵ��װ�á��ش��������⣺
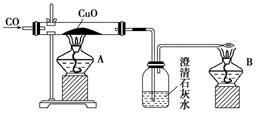
(1)ʵ��ʱӦ�ȵ�ȼ________(�A����B��)���ľƾ��ơ�
(2)ϴ��ƿ�е�����Ϊ__________________________________________________________��
(3)�ƾ���B��������_________________________________________________________��
(4)��ͬѧ��������̫���ӣ��ɽ��ƾ��ƺ϶�Ϊһ��ȥ��B����β�����ܿ���ת��A�Ļ����ϼ��ɣ���ͬѧ����Ƿ������________��������______________________________��
(5)��ͬѧ����CO�ܷ�ʹʯ��ˮ����ǣ�����������COͨ��CuO֮ǰӦ��ͨ������ʯ��ˮ���ų�CO�����ʯ��ˮ��Ӧ���ԶԴ��������ۣ�����Ϊ�������________(���Ҫ������Ҫ��)��������____________________________________________��
(6)��ͬѧ��Ϊ����Ƶ�װ����β����������������������������Ϊ��ͬѧ���һ�ֺ�����β������������________________________________________��
(1)B
(2)����ʯ��ˮ�����
(3)��δ��Ӧ��COת��ΪCO2����ֹ��Ⱦ����
(4)��������A��B����ͬʱ��ȼ
(5)����Ҫ���ڵ�ȼA֮ǰCO��ͨ��ʯ��ˮ
(6)�ڵ���ĩ������һ����(������ƿ)�ٴ���
������������Ĺؼ��Ǹ���ʵ��ԭ��������ʵ���������������CO�ǿ�ȼ�����壬�������ϼ��ȿ��ܷ�����ը�������CuO��Ӧǰ��Ӧ��ͨ��CO�ų�װ���еĿ�����CO�ж�����Ӧ�ȵ�ȼB���ƾ��ƽ��д������ڷ�ӦǰCO��ͨ��ʯ��ˮ��û��Ҫ�����CO��ʯ��ˮ�ķ�Ӧ��

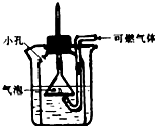 ��ͼ��ijͬѧ��Ƶ�һ����ȼ���尲ȫ��ȼװ�ã��Դ�װ�õ����ۺ�ʹ�ô�����ǣ�������
��ͼ��ijͬѧ��Ƶ�һ����ȼ���尲ȫ��ȼװ�ã��Դ�װ�õ����ۺ�ʹ�ô�����ǣ�������| A���ô�װ�õ�ȼ��ȼ������ɷ�ֹ��ը�ķ��� | B��ʵ��ʱ�ձ���ˮ���Ķ��ٲ���Ӱ��ʵ��Ч�� | C����װ�÷�ֹ���屬ը��ԭ����ʹ���������岻��ȼ������ֱ�ӽӴ� | D����װ��������������ˮ�Ŀ�ȼ������ |
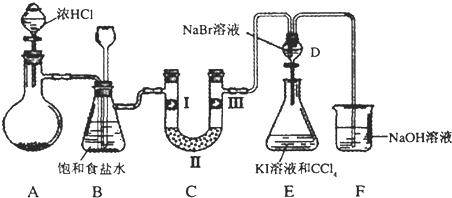
��1����װ��A���Ʊ�����ѡ�õ�ҩƷΪ����������̺�Ũ���ᣬ��д��װ��A�еĻ�ѧ��Ӧ����ʽ��
��2��װ��B�б���ʳ��ˮ��������
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η���
| a | b | c | d | |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | ��ʯ�� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��5��װ��F����������NaOH��Һ�������ȣ���д����Ӧ�����ӷ���ʽ
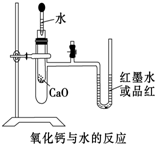 ��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ��˳���ǣ�
��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ��˳���ǣ�